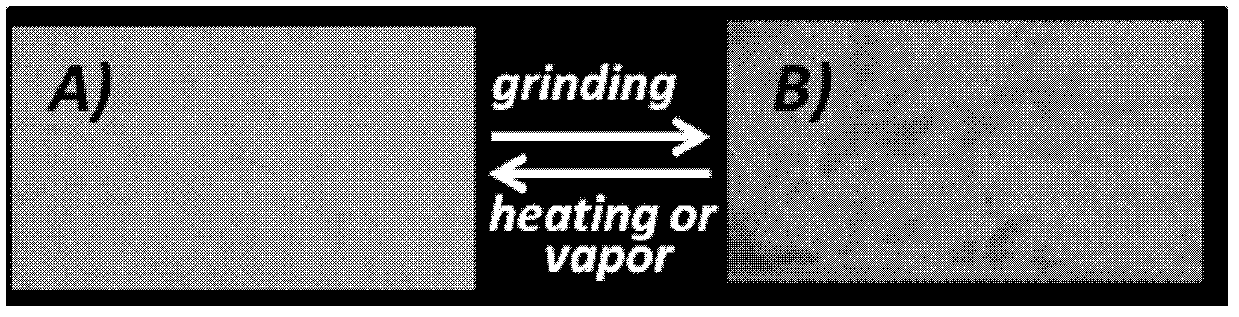
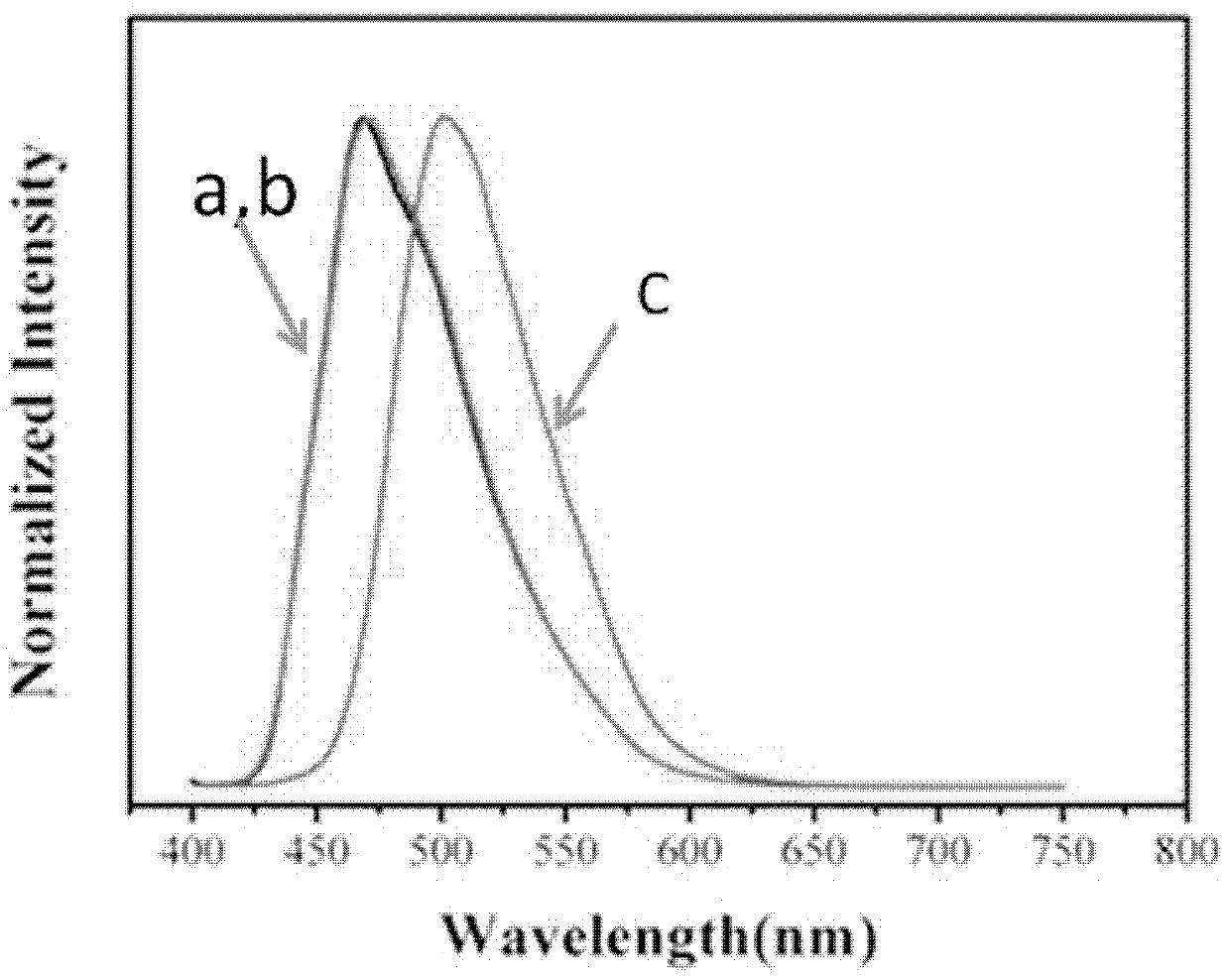
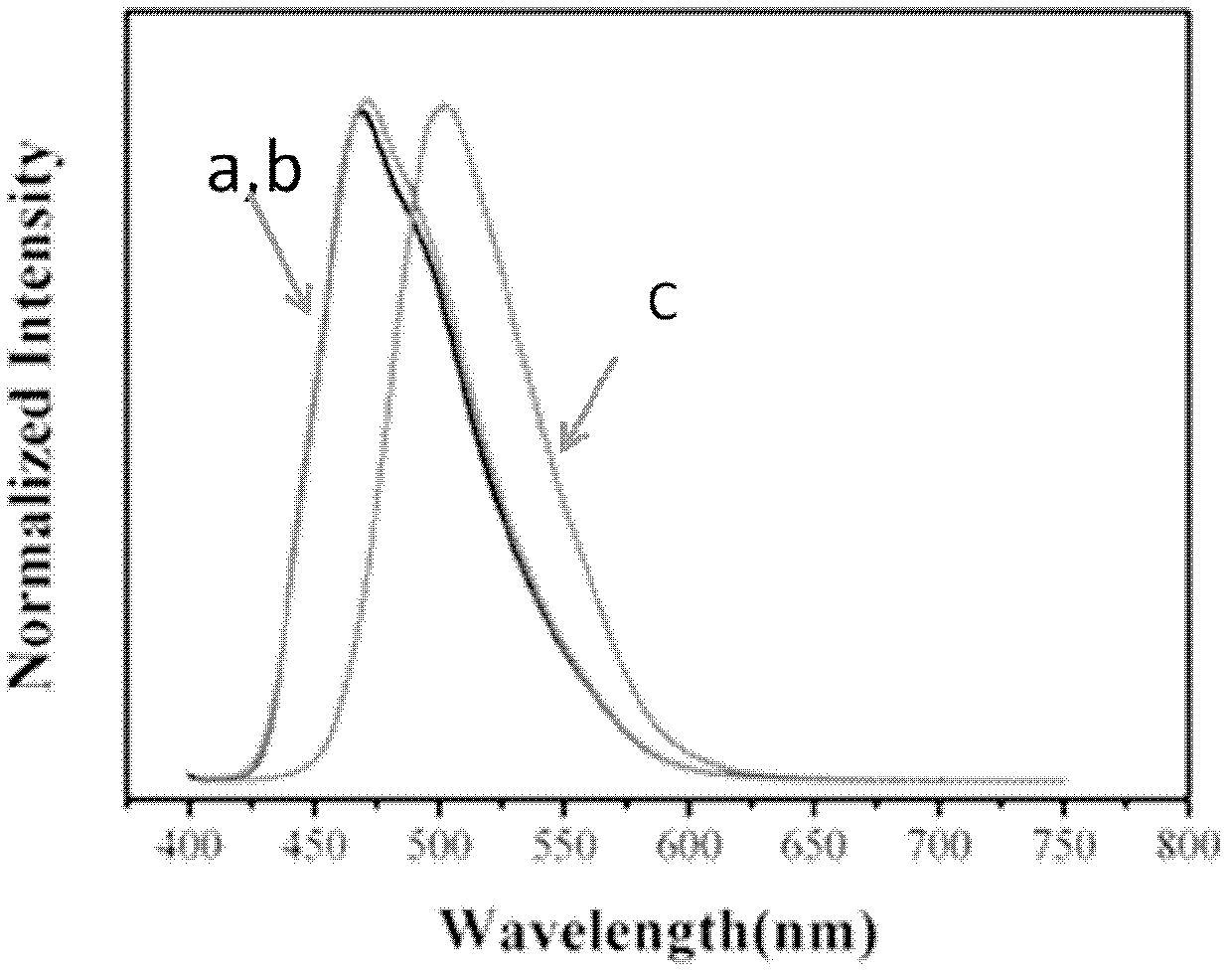
Stilbene nitrile derivatives, and preparation method and application thereof
A stilbene nitrile and derivative technology, which is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of low reversible temperature, limited application, harmful to the body, etc., and achieves a simple and convenient preparation process. The effect of convenient device preparation and easy color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 4 g (20 mmol) of p-bromonitrile, 3.26 g (12 mmol) of p-methoxybenzaldehyde and sodium methoxide (0.12 g, 2 mmol) were dissolved in 30 ml of chromatographically pure ethanol. Stir the reaction at room temperature and terminate the reaction when there are a large number of solid particles. Then put it into the refrigerator overnight and filter, and the filter cake was washed three times with ethanol to obtain 5.66 g of white powder brominated stilbene nitrile intermediate (IV), with a yield of 90%. Results of substance structure tests: 1 H NMR (500MHz, CDCl 3 ) δ (ppm) 7.78(s, 1H), 7.48(d, J=7.5Hz, 2H), 7.39(d, J=7.5, 2H), 7.34(d, J=7.5, 2H), 6.91(d, J=7.5, 2H), 3.82(s, 1H), ; 13 C NMR (500MHz, CDCl 3 ); δ161.3, 141.2, 132.7, 131.8, 131.0, 126.1, 119.7, 114.5, 106.6, 56.0; MS (EI): m / e 313.0 (M + ).
Embodiment 2
[0044] 1.96 g (10 mmol) of p-bromonitrile benzyl, 1.09 g (8 mmol) of p-methoxybenzaldehyde and sodium methoxide (0.03 g, 0.5 mmol) were dissolved in 30 ml of chromatographically pure ethanol. The reaction was stirred at room temperature until a large number of solid particles terminated the reaction. Then put it in the refrigerator overnight and filter, and rinse the filter cake with ethanol three times to obtain 2.20 g of white powder brominated stilbene nitrile intermediate (IV), with a yield of 70%.
Embodiment 3
[0046] 1.96 g (10 mmol) of p-bromonitrile benzyl, 2.72 g (20 mmol) of p-methoxybenzaldehyde and sodium methoxide (0.03 g, 0.5 mmol) were dissolved in 30 ml of chromatographically pure ethanol. The reaction was stirred at room temperature until a large number of solid particles terminated the reaction. Then put it into the refrigerator overnight and filter, and the filter cake was washed three times with ethanol to obtain 2.98 g of white powder brominated stilbene nitrile intermediate (IV), with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com