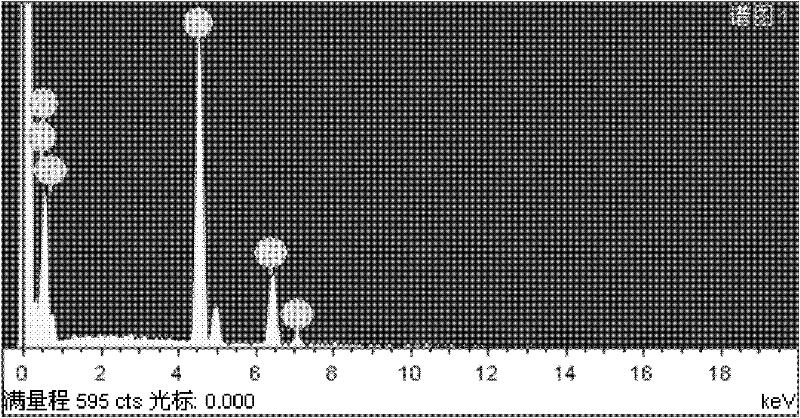
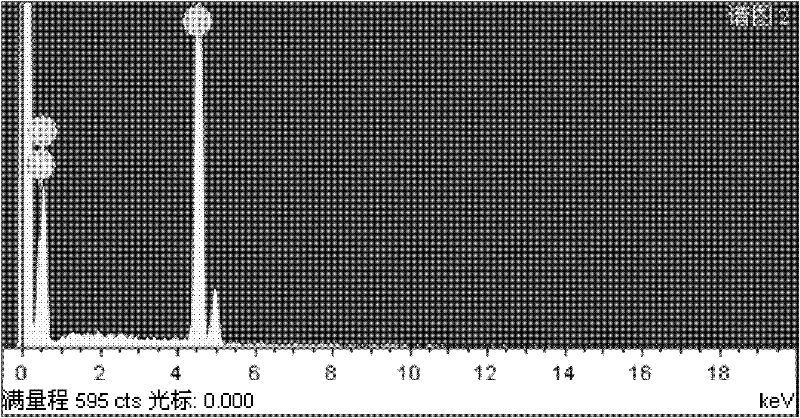
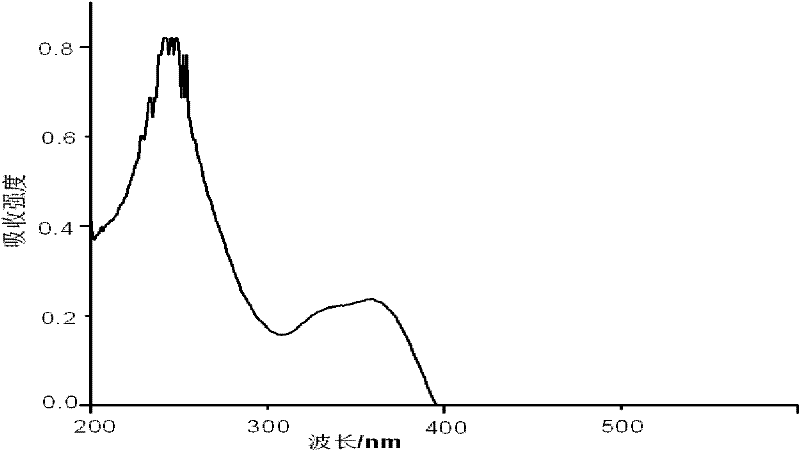
Process for preparing iron-doped titanium dioxide powders
A preparation process and technology of titanium dioxide, which are applied in semiconductor/solid-state device manufacturing, photosensitive equipment, electric solid-state devices, etc., can solve the problems of unsuitable solar cell electrode materials, poor ability to capture sunlight, and low utilization rate of sunlight, so as to improve the Photoelectric conversion efficiency, slowing down the rate of hydrolysis, and extending the sensing range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Slowly drop 10ml of butyl titanate into a 50ml beaker filled with 10ml of absolute ethanol under stirring. After the dropwise addition, continue to drop 1ml of glacial acetic acid under stirring, and stir vigorously for 30 minutes to obtain a uniform and transparent solution A .
[0034] (2) Weigh 0.49g FeCl 3 ·6H 2 O, the molar ratio of Fe and Ti is 20:1, add 5ml of ethanol, dissolve at room temperature, and obtain FeCl-containing 3 solution B.
[0035] (3) At room temperature, solution B was slowly added dropwise to solution A, and was added dropwise with stirring to obtain uniform and transparent solution C.
[0036] (4) After stirring for 30 minutes, stop stirring, and leave the obtained solution C at room temperature for 2 days to obtain a yellow wet gel. After naturally standing at room temperature for 72 hours, put it into an oven with a set temperature of 110°C for drying .
[0037] (5) Grind the obtained xerogel into a powder with uniform particles, pl...
Embodiment 2
[0044] (1) Slowly drop 10ml of butyl titanate into a 50ml beaker filled with 10ml of absolute ethanol under stirring. After the dropwise addition, continue to drop 1.0ml of glacial acetic acid under stirring, and stir vigorously for 30 minutes to obtain a uniform and transparent solution. A;
[0045] (2) Weigh 0.98g FeCl 3 ·6H 2 O, the molar ratio of Fe and Ti is 10:1, add 5ml of ethanol, dissolve at room temperature, and obtain FeCl-containing 3 solution B.
[0046] (3) At room temperature, slowly add solution B dropwise to solution A, and add dropwise under stirring to obtain uniform and transparent solution C.
[0047] (4) After stirring for 15 minutes, stop stirring, and leave the obtained solution C at room temperature for 2 days to obtain a yellow wet gel. After naturally standing at room temperature for 72 hours, put it into an oven with a set temperature of 110°C for drying .
[0048] (5) Grind the obtained xerogel into a powder with uniform particles, place it in...
Embodiment 3
[0051] (1) Slowly drop 10ml of butyl titanate into a 50ml beaker filled with 10ml of absolute ethanol under stirring. After the dropwise addition, continue to drop 1ml of glacial acetic acid under stirring, and stir vigorously for 30 minutes to obtain a uniform and transparent solution A .
[0052] (2) Weigh 1.96g FeCl3·6H2O, the molar ratio of Fe and Ti is 5:1. Add 5ml of ethanol and dissolve at room temperature to obtain solution B containing FeCl3.
[0053] (3) At room temperature, slowly add solution B to solution A drop by drop while stirring, and all the drops are completed to obtain uniform and transparent solution C, and continue to stir.
[0054] (4) After stirring for 20 minutes, stop stirring, and leave the obtained solution C at room temperature for 2 days to obtain a yellow wet gel. After naturally standing at room temperature for 72 hours, put it into an oven with a set temperature of 110°C for drying .
[0055] (5) Grind the obtained xerogel into a powder wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com