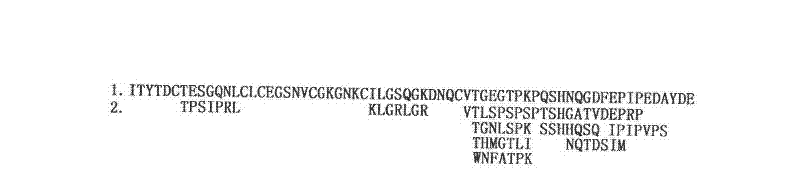Preparation of low-immunogenicity recombinant hirudin mutant
A recombinant hirudin, immunogenic technology, applied in the preparation method of peptides, from leech inhibitors, protease inhibitors, etc., can solve the problems of affecting clinical drug effect, inactivation of hirudin, loss of pharmacological activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
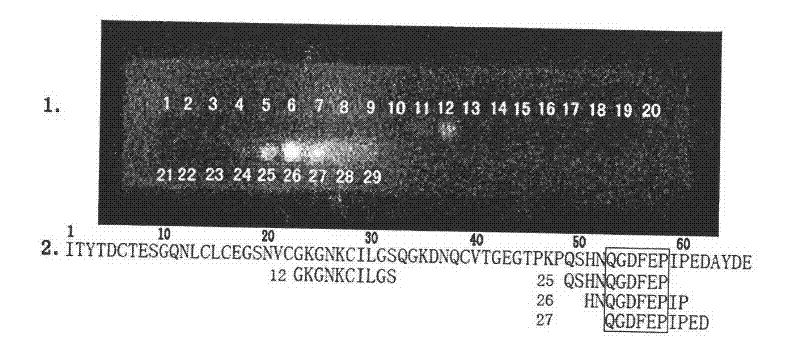
[0010] The identification of embodiment 1 recombinant hirudin III epitope
[0011] Using Ph.D. from New England Biolabs TM -7 Phage Display Peptide Library Kit for epitope panning. First, the plate was embedded with an appropriate dilution of guinea pig anti-rHV3 antibody and incubated overnight. Each plate was filled with blocking solution, reacted at 4°C for at least 1 hr, and the plate was quickly washed 6 times with TBST (TBS+0.1% [v / v] Tween-20) buffer. Dilute 2 x 10 with 1 ml of TBST buffer 11 The phage (that is, 10 μl of the original library) was then added to the coated plate and shaken gently at room temperature for 10-60 min. Pour to remove unbound phage, wash the plate 10 times with TBST buffer. Competitive elution was performed with 1 ml of 0.1-1 mM rHV3 in TBS solution. The eluate was spliced into an overnight culture of E. coli ER2738. Incubate vigorously at 37°C for 4.5 hr, centrifuge, and take the supernatant. Add 1 / 6 volume of PEG / NaCl solution, preci...
Embodiment 2
[0016] Example 2 Alanine (Ala) scanning technology determines the key binding residues of dominant antigenic epitopes
[0017] 1. According to the principle of overlap extension PCR site-directed mutagenesis ( Figure 6 ), designed a pair of common outer primers and 6 pairs of mutation primers (Table 1) with the plasmid pTASH (Tan, S., et al. (2002). Protein Expression and Purification, 25, 430-436.) as a template, the first Amino acid residues 53, 54, 55, 56, 57, and 58 were point-mutated into Ala respectively. The specific operation is as follows: use the outer primer f and each mutation primer r to amplify fragment A by PCR, then use each mutation primer f and outer primer r to amplify fragment B, then mix fragments A and B, and use this mixture As a template, the outer primer f and the outer primer r are used to carry out PCR, thereby obtaining gene C fragments in which the 53rd, 54th, 55th, 56th, 57th, and 58th amino acid residues are mutated.
[0018] Table 1. Primer s...
Embodiment 3
[0028] Embodiment 3 animal experiments identify the immunogenicity of Q53A hirudin mutants
[0029]Take 20 guinea pigs, half male and half male, and randomly divide them into 2 groups, ie wild-type experimental group and mutant experimental group. Wild-type hirudin and Q53A hirudin mutant were administered subcutaneously on the back, administered twice a day, dose 1.6mg / kg / day, blood was taken after 10 days of administration, and the antibody titers of the two groups of animal serum were detected by ELISA ( Figure 5 ). The results showed that the immunogenicity of the Q53A hirudin mutant was significantly reduced.
[0030] The results of the present invention show that the Q53A hirudin mutant basically maintains the original anticoagulant specific activity, but its immunogenicity is significantly reduced, overcomes the immunogenicity problem of existing hirudin drugs, improves the clinical drug effect of hirudin drugs, and fully It is of great significance to exert its clin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com