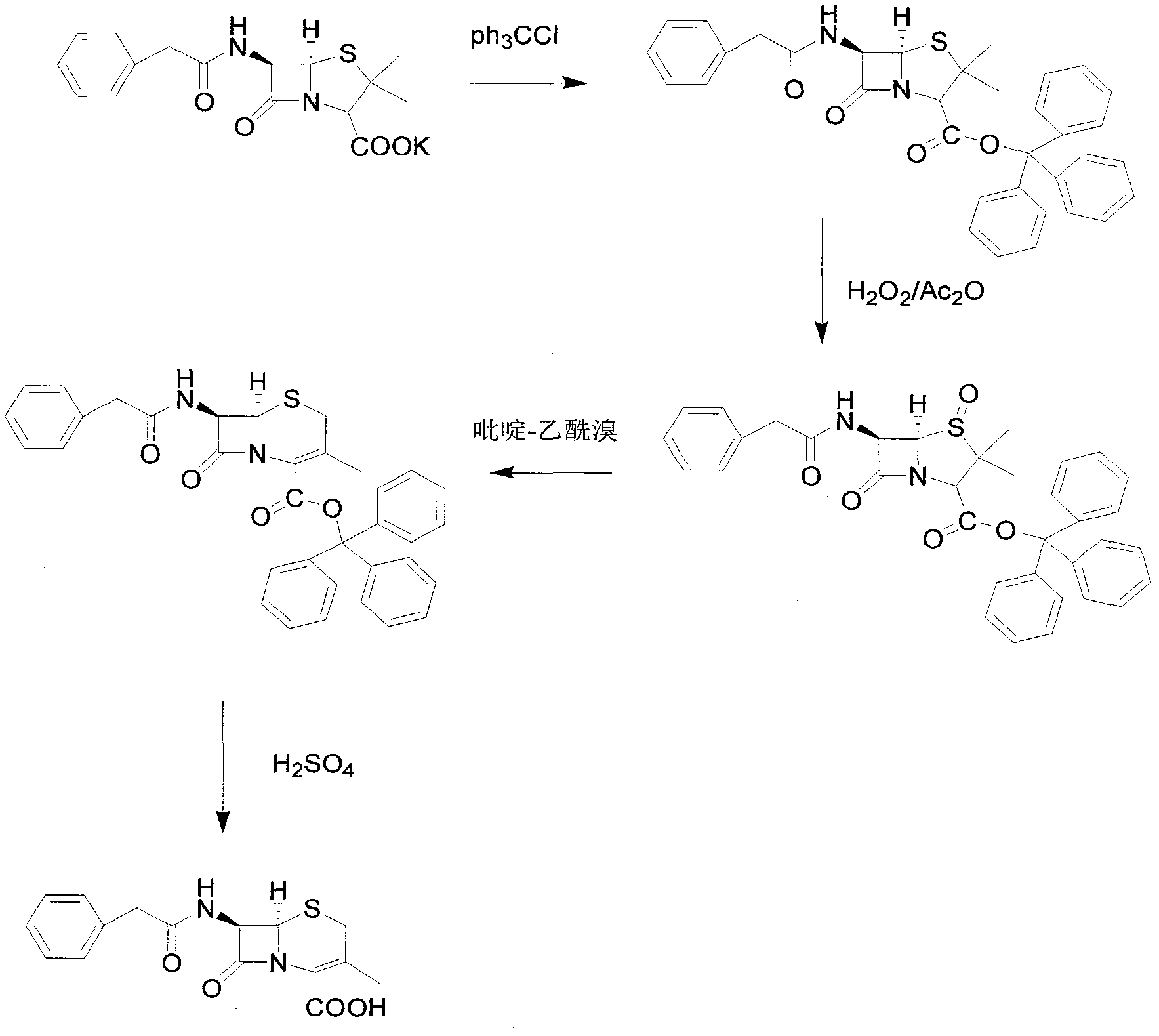
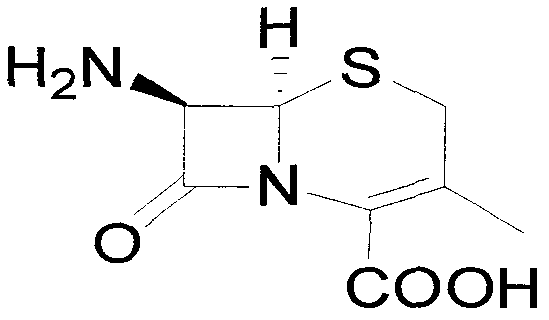
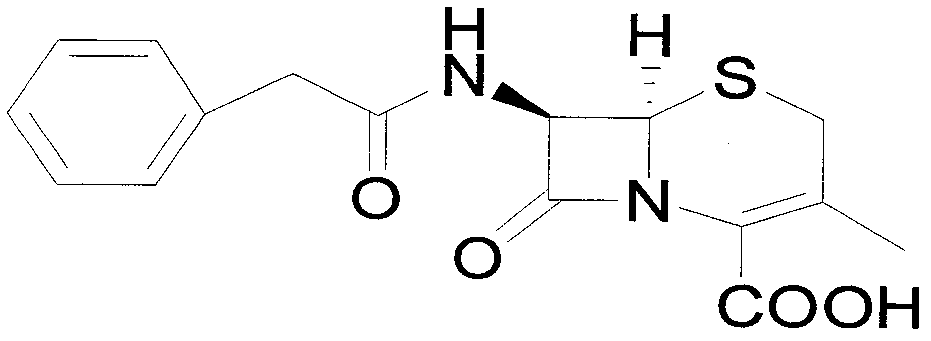
Preparation method of 7-aminodeacetoxy cephalosporanic acid
A technology of acetoxycephalosporanic acid and phenylacetamido, which is applied in the field of preparation of 7-phenylacetylaminodesacetoxycephalosporanic acid, can solve problems such as high cost, influence on yield, difficult control of side reactions, etc., and achieve The effect of short production cycle, less discharge of three wastes and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 372.5g of penicillin G potassium and 1860g of toluene to the reaction flask and stir to form a suspension, add 305.5g of triphenylchloromethane and 63.3g of tetrabutylammonium bromide, heat up to 50°C for reaction, and monitor the progress of the reaction by TLC. After the reaction was completed, the temperature was lowered to about 5°C, a solution made of 104.3g potassium acetate, 1.9g acetic acid and 930g deionized water was added, and 96.9g (1.4mol) of 50% potassium acetate was added dropwise at a temperature of 0-5°C. Hydrogen peroxide and 141.6g acetic anhydride. After the dropwise addition, react at 0-5°C, and monitor the progress of the reaction by TLC. After the reaction was completed, the organic layer was separated and washed twice with 930 g of water. After the organic layer was refluxed to separate water for 2 hours, the temperature was lowered to about 80° C., and 201.2 g of a ring expansion catalyst pyridine-acetyl bromide solution in toluene with a c...
Embodiment 2
[0044] Add 1,000 g of penicillin G potassium and 5,000 g of toluene to the reaction flask and stir to form a suspension, add 780 g of triphenylchloromethane and 140 g of tetrabutylammonium bromide, heat up to 50°C for reaction, and monitor the progress of the reaction by TLC. After the reaction is completed, filter and cool the filtrate to about 5°C. After adding a solution made of 290g of potassium acetate, 3g of acetic acid and 2000g of deionized water, add 240g of 50% hydrogen peroxide and 360g of hydrogen peroxide and 360g of Acetic anhydride. After the dropwise addition, react at 0-5°C, and monitor the progress of the reaction by TLC. After the reaction was completed, the organic layer was separated and washed twice with 2000 g of water. After the organic layer was refluxed to separate water for 2 hours, the temperature was lowered to about 60° C., and 460 g of 20% ring expansion catalyst pyridine-acetyl bromide in toluene was added. After the addition was completed, th...
Embodiment 3
[0046] Add 700g of penicillin G potassium and 3500g of toluene to the reaction flask and stir to form a suspension, add 574g of triphenylchloromethane and 91g of tetrabutylammonium bromide, heat up to 60°C for reaction, and monitor the progress of the reaction by TLC. After the reaction is completed, filter, and the filtrate is cooled to about 5°C. After adding a solution made of 203g potassium acetate, 3.5g acetic acid and 2100g deionized water, 154g concentration of 50% hydrogen peroxide and 50% hydrogen peroxide and 287 g of acetic anhydride. After the dropwise addition, react at 0-5°C, and monitor the progress of the reaction by TLC. After the reaction was completed, the organic layer was separated and washed twice with 1400 g of water. After the organic layer was refluxed to separate water for 2 hours, the temperature was lowered to about 70° C., and 287 g of a ring expansion catalyst pyridine-acetyl bromide solution in toluene with a concentration of 20% was added. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com