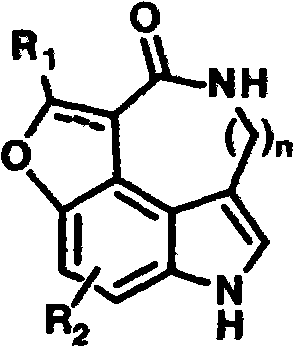
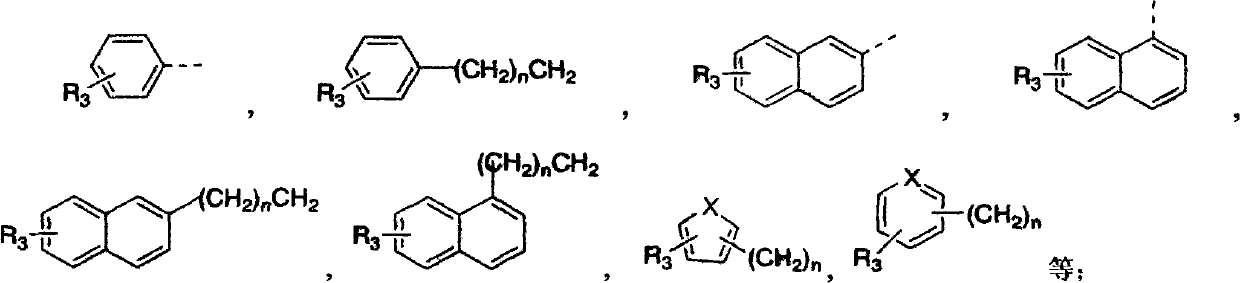
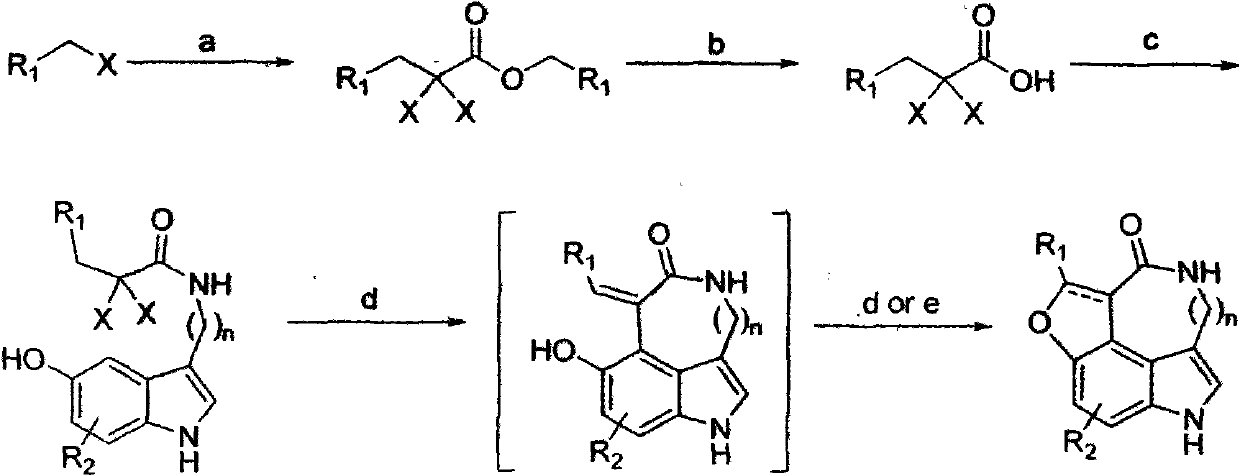
Preparation of substituted indole lactam derivative and application of substituted indole lactam derivative as antimalarial agent
A technology for products and compounds, applied in the field of biomedicine, can solve the problems of inability to obtain analogs effectively, long steps, and difficult synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Compound 1: In tetrahydrofuran (3 mL) solvent, add the corresponding bromide (642 mg, 3.0 mmol), sodium trichloroacetate (835 mg, 4.5 mmol) and tetrabutylammonium bromide (96 mg, 0.3 mmol). The mixture was reacted at 60°C for 24 hours. Then the mixture was added with water and extracted with ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate, filtered, concentrated, and then separated by column chromatography to obtain the corresponding compound 1 (713 mg, 60%).
[0035] 1 H NMR(400MHz, CDCl 3 )d 6.69-6.88(m, 6H), 5.98(s, 2H), 5.94(s, 2H), 5.17(s, 2H), 3.62(s, 2H);
[0036] 13 C NMR(100MHz, CDCl 3 )d165.5, 148.1, 147.9, 147.4, 147.3, 127.9, 126.6, 124.7, 122.8, 111.3, 109.2, 108.3, 107.9, 101.3, 101.1, 84.0, 69.3, 50.2;
[0037] HRMS(ESI)m / z calcd for C 18 H 14 Cl 2 NaO 6 (M+Na) + 419.0060; found 419.0067.
Embodiment 2
[0039]
[0040] Compound 2: Dissolve compound 1 (486mg, 1.0mmol) in H 2 O / THF (1 / 3, 12 mL), then NaOH (120 mg, 3.0 mmol) was added. The mixture was reacted at room temperature for 3 hours. Then spin off part of the solvent, add 50ml of water to the remaining mixture; then wash twice with dichloromethane, discard the dichloride layer, the resulting aqueous layer is acidified with 1M hydrochloric acid solution to make the solution pH less than 5, and then add ethyl acetate After extraction, the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and the organic solvent was removed by rotating to obtain the desired product 2 (249 mg, 95%). Can be used directly without further purification.
[0041] 1 H NMR(400MHz, acetone-d 6 )d 6.91 (d, J = 1.6 Hz, 1H), 6.88 (dd, J = 8.0, 1.6 Hz, 1H), 6.80 (d, J = 8.0 Hz, 1H), 5.99 (s, 2H), 3.69 (s , 2H);
[0042] 13 C NMR(100MHz, acetone-d 6 )d167.9, 149.1, 149.0, 129.2, 126.5, 113.0, 109.4, 102.9, 86.8, 51.4;
[0043] HRM...
Embodiment 3
[0045]
[0046] Compound 3: Compound 2 (157mg, 0.6mmol) was dissolved in treated DMF / CH 2 Cl 2 (1 / 5, 6mL) solution, then add HOBt (88mg, 0.65mmol), HBTU (246mg, 0.65mmol), 5-hydroxytryptamine hydrochloride (106mg, 0.5mmol) and DIPEA in ice bath conditions (134μL / 0.75mmol). The reaction solution was gradually warmed to room temperature, and then stirred at room temperature for 24 hours. Part of the solvent was spun off under reduced pressure, then diluted acidic water was added, then extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, concentrated, and then separated by column chromatography to obtain the desired compound 3 (185 mg, 88%).
[0047] 1 H NMR(400MHz, acetone-d 6 )d 9.74 (br s, 1H), 7.93 (br s, 1H), 7.65 (s, 1H), 7.19 (d, J=8.8Hz, 1H), 7.01 (d, J=2.4Hz, 1H), 7.00 (d, J = 2.4 Hz, 1H), 6.89 (d, J = 1.6 Hz, 1H), 6.84 (dd, J = 8.0, 1.6 Hz, 1H), 6.79 (d, J = 8.0 Hz, 1H), 6.71 (dd, J=8.8, 2.4 Hz, 1H), 5.97 (s, 2H), 3.69 (s, 2H), 3.52 (m, 2H), 2.86 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com