Method for preparing dinitrogen heterocyclooctatetraene
A technology of cyclooctatetraene and diazepine, which is applied in the field of organic synthesis, can solve problems such as harsh preparation conditions, easy polymerization, and expensive products, and achieve the effects of simplifying the difficulty of synthesis conditions, reducing one-step reactions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
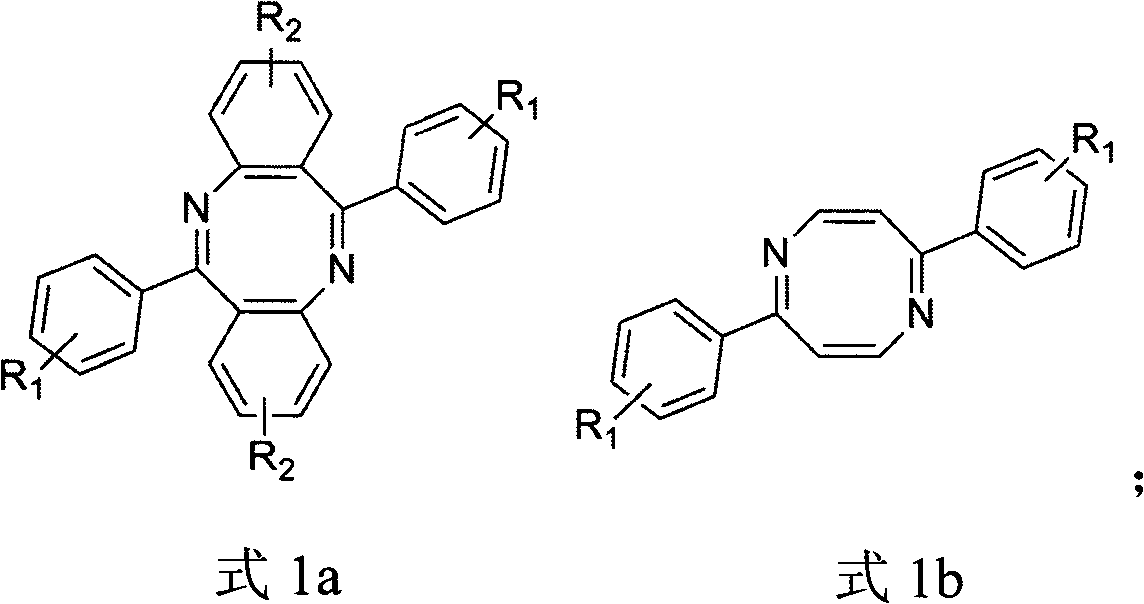
[0018] The method that the present invention proposes to prepare diazacyclooctene is as follows:
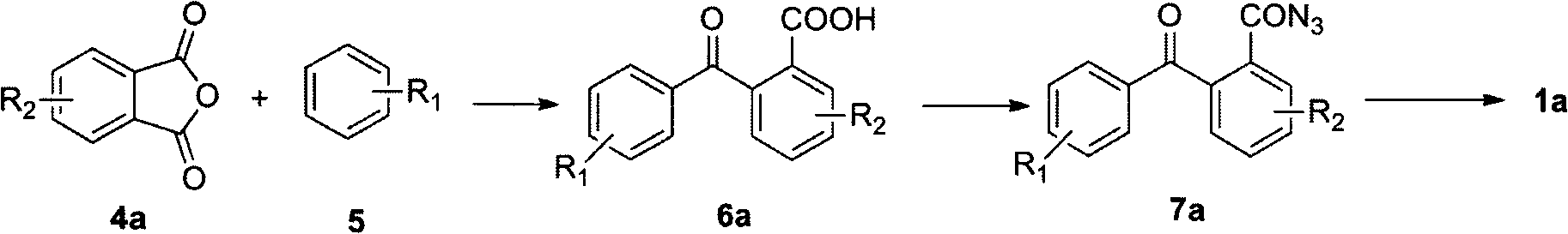
[0019] 1) Preparation of substituted benzoylbenzoic acid: using anhydrous aluminum chloride as Friedel-Crafts reagent, anhydride reacts with aromatic compound to replace benzoylbenzoic acid;
[0020] 2) Preparation of benzoyl azide: first, benzoyl chloride is prepared by using substituted benzoyl benzoic acid under the condition of chlorinating reagent; then the corresponding acyl azide is prepared from benzoyl chloride and azide salt.
[0021] 3) Preparation of diazaoctene: the acyl azide compound is subjected to a ring closure reaction at a certain temperature under the condition of an acidic reagent to prepare diazaoctatetraene.
Embodiment 1
[0023] In a 500mL three-necked round-bottomed flask equipped with a thermometer, a stirrer, and a nitrogen inlet tube, add 200 ml of benzene (5) (R 1 =H) and 50 grams of phthalic anhydride (4a) (R 2 =H), 118 grams of aluminum trichloride, stirred at room temperature for 2 hours, then raised the temperature to 50 degrees Celsius, and reacted with stirring for 5 hours. The reaction was terminated, cooled to room temperature, and poured into a large amount of ice water. Extract with dichloromethane, concentrate the solvent under reduced pressure, and the resulting product is recrystallized with ethanol to obtain o-benzoylbenzoic acid (6a) (R 1 , R 2 =H), yield 85%.
[0024] Phthaloylbenzoic acid (6a) (R 1 , R 2 = H) 3.0 g, 20 ml of thionyl chloride, reflux for 4 hours, concentrate under reduced pressure to prepare o-benzoylbenzoyl chloride; add 30 ml of anhydrous tetrahydrofuran to the system, stir to dissolve and put it in an ice bath; configure Add 5 ml of an aqueous solu...
Embodiment 2
[0035] With embodiment 1, used benzene compound is bromobenzene, 6,12-bis (4'-bromophenyl) dibenzo (b, f) diazacyclooctatetraene (1a) (R 1 =Br,R 2 =H), the overall yield was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com