Novel vinylidene fluoride copolymers and processes for production thereof
A technology of vinylidene fluoride and a manufacturing method, applied in 1 field, can solve problems such as defective heat resistance and reduced commercial value, and achieve the effects of good adhesion and excellent pollution resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] [Example 1] (1,1-difluoroethylene / dimethylmethylenemalonate=99 / 1 (mass ratio))
[0121] 1024 g of ion-exchanged water, 0.6 g of methylcellulose, 1.2 g of ethyl acetate, 8.6 g of 50% by weight diisopropyl peroxydicarbonate-chlorofluorocarbon 225cb solution, 1, 396 g of 1-difluoroethylene (VDF), 4.0 g of dimethyl methylene malonate, and suspension polymerization were performed at 29° C. for 22 hours until the pressure dropped to 1.5 MPa. After the polymerization was completed, the polymer slurry was heat-treated at 95° C. for 30 minutes to deactivate the polymerization initiator, and the polymer was filtered, dehydrated and washed with water, and then dried at 80° C. for 20 hours to obtain a polymer powder. The yield was 86%, and the inherent viscosity of the obtained polymer was 1.29 dl / g.
Embodiment 2~4、 comparative example 1~3
[0123] In Examples 2 to 4 and Comparative Examples 1 to 3, except that the ratio of 1,1-difluoroethylene (VDF) to comonomer and the type of comonomer were changed as described in Table 1, the same method as in Example was adopted. 1 in the same way. In addition, Comparative Example 1 is a homopolymer of vinylidene fluoride.
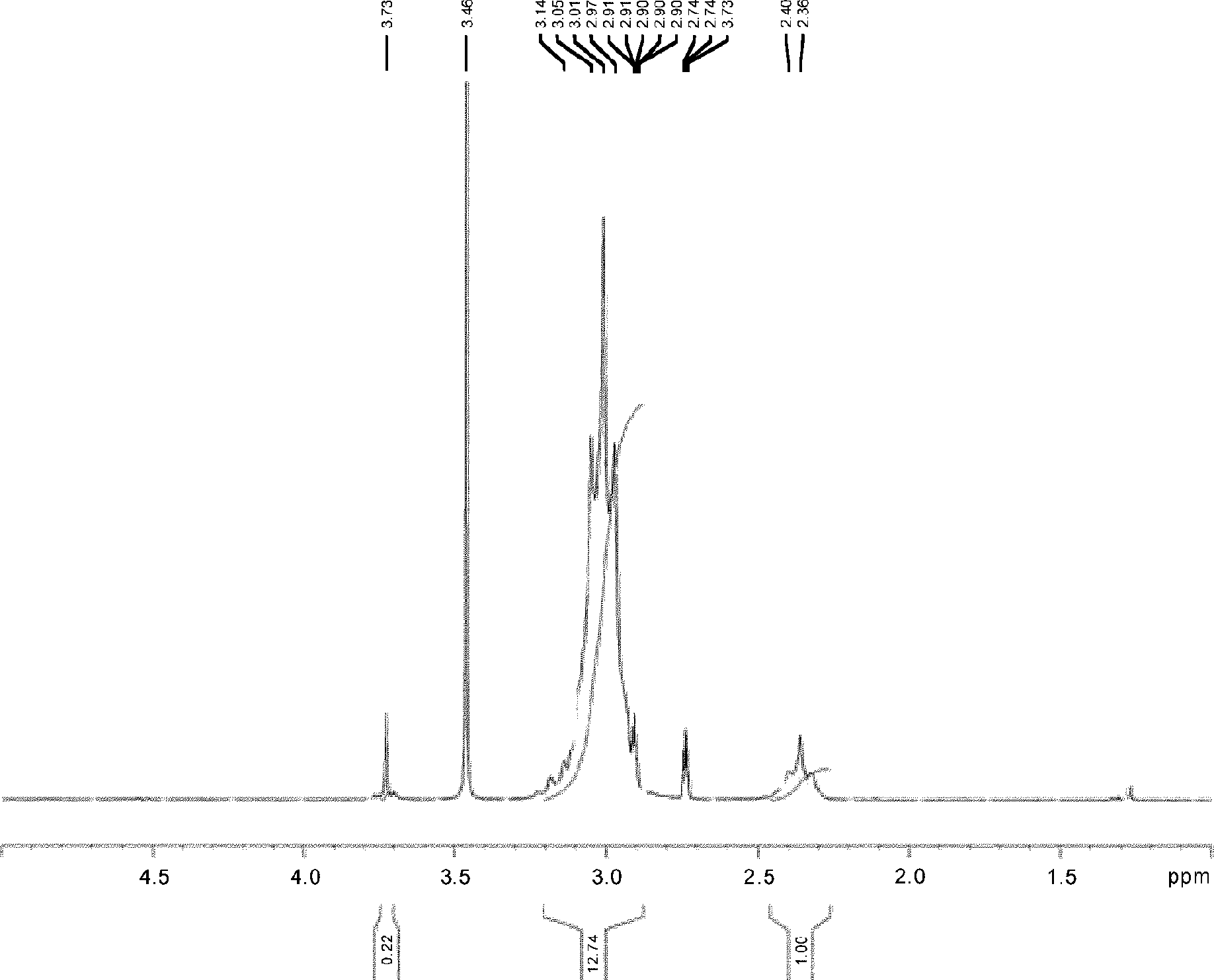
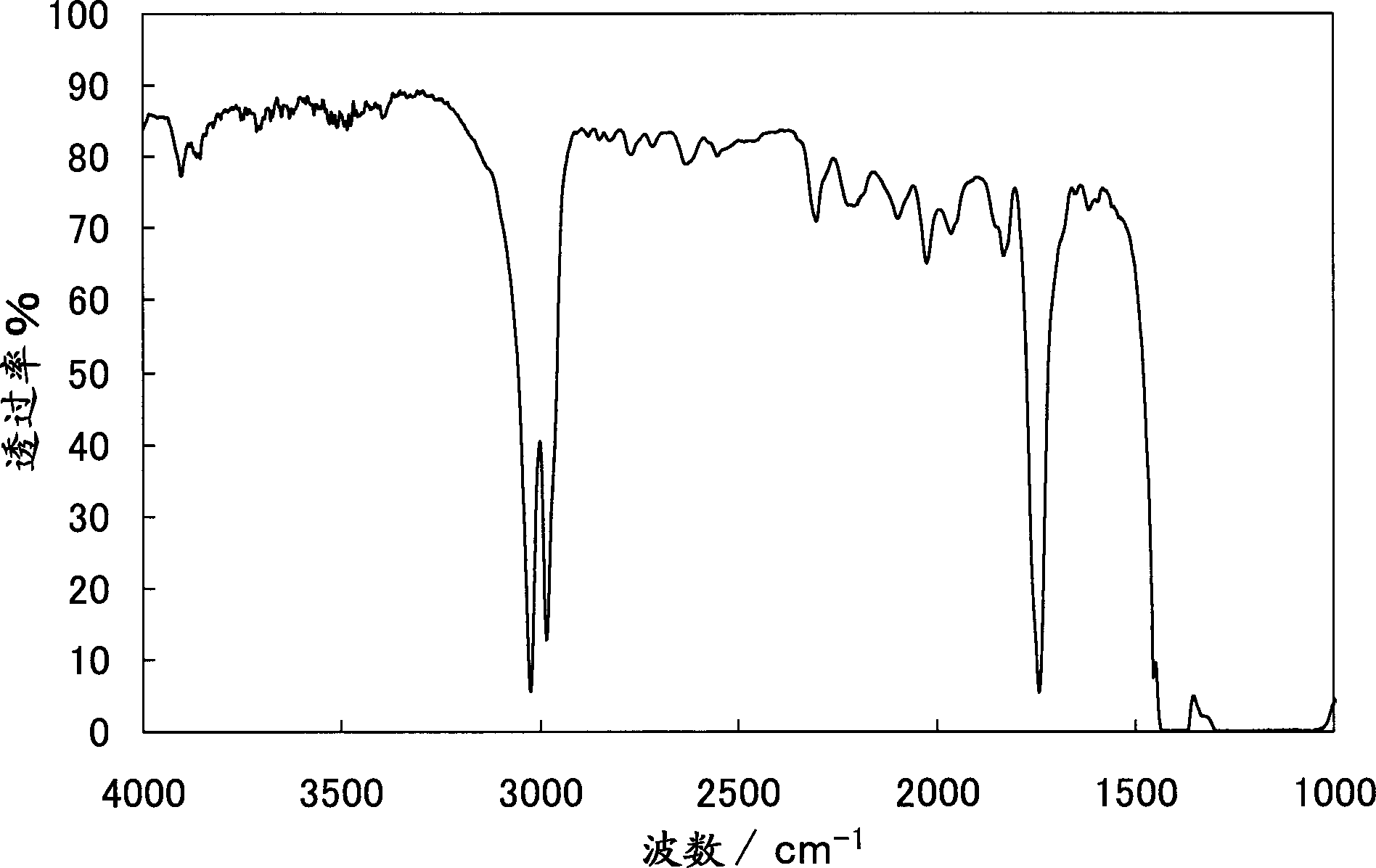
[0124] Furthermore, regarding Example 2, as figure 2 and image 3 shown by the resulting polymer in the 1 In the H-NMR spectrum, it was observed around 3.7ppm that originated from COOCH 2 The signal of R (R=H in embodiment 1,2,4, comparative example 2,3, R=Me in embodiment 3), and in IR spectrum in 1740cm -1 A signal derived from C=O stretching vibration was observed nearby, confirming that a copolymer of methylene malonate was obtained. pass 1 The area ratio in the H-NMR spectrum calculates the ratio (molar ratio) of the vinylidene fluoride unit to the vinylidene malonate unit contained in the vinylidene fluoride copolymer. In addition, the ratio...
Embodiment 5
[0140] 125 ml of N,N-dimethylformamide and 1.17 g of lithium bromide were added to a 500 ml three-necked flask equipped with a magnetic stirring bar and a Dimro cooling tube to prepare a solution. To this solution was added 5.05 g of the 1,1-didene fluoride copolymer of the present invention obtained in Example 2, followed by heating and stirring at 100° C. for 9 hours. After naturally cooling to room temperature, the reaction solution was reprecipitated using dilute hydrochloric acid. The resulting solid was filtered and fractionated, dehydrated and washed with water, and then dried at 80° C. for 20 hours. Measure the resulting solid 1 H-NMR spectrum, results attributed to COOCH 3 The change of the integrated value of the signal (near 3.7ppm) confirms that about 41% of the total number of ester groups in the copolymer is hydrolyzed (refer to Figure 5 and Image 6 ).
[0141] [table 3]
[0142] Mn / 10 5 Mw / 10 5 Mn / Mw Tm(°C) Tc(°C) Example 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com