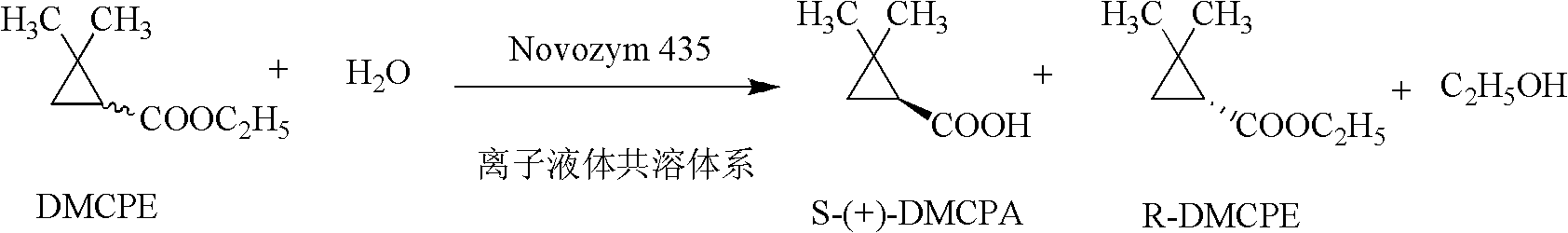
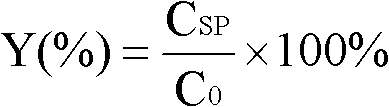Method for preparing (S)-(+)-2,2-dimethyl cyclopropane methanoic acid by biological resolution in ionic liquid cosolvent
A technology of ethyl dimethylcyclopropanecarboxylate and dimethylcyclopropane, which is applied in the field of biological separation and preparation-2, can solve the problems of easy agglomeration, high viscosity and low solubility, and achieves good stability , good dispersibility, the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Add hydrophilic ionic liquid 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim]OTF) to increase substrate solubility, improve the substrate concentration and enzyme-catalyzed resolution of the reaction Reaction efficiency of ethyl 2,2-dimethylcyclopropanecarboxylate.
[0026] Dissolve 0.1ml of [Bmim]OTF in 9.9ml of pH 7.2, 1mol / L phosphate buffer, add 65mmol of substrate ethyl 2,2-dimethylcyclopropanecarboxylate, add 160mg of lipase Novozym 435, Enzymatically catalyzed reactions were carried out in 50ml Erlenmeyer flasks. The reaction temperature was 30° C., the rotation speed of the shaker was 200 r / min, and the reaction was performed for 24 hours. After the reaction, extract with twice the volume of ethyl acetate, set the volume to 20ml, and perform gas chromatography analysis. The concentration of the product obtained is 2.8g / L, the ee value of the product is 97.8%, and the yield is 38.1%.
Embodiment 2~7
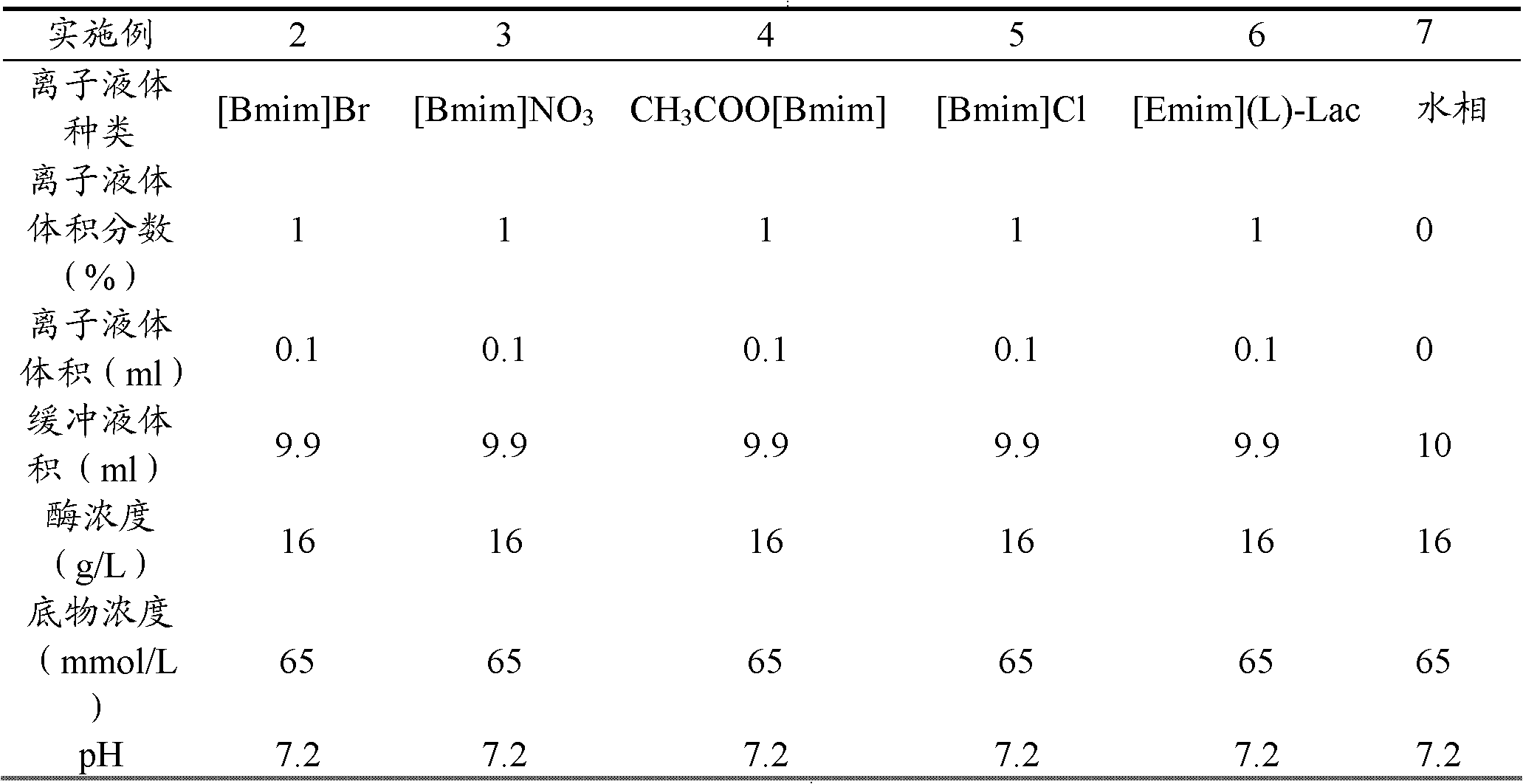
[0028] With reference to the method of Example 1, the reaction of the aqueous phase system under the same conditions is used as a contrast (i.e. the reaction medium is 10ml of pH 7.2, 1mol / L phosphate buffer), to investigate the effect of different types of hydrophilic ionic liquids on 2,2- The impact of ethyl dimethylcyclopropane formate bioresolution, the results are shown in Table 1:
[0029] Table 1
[0030]
[0031]
[0032] Conclusion: The above hydrophilic ionic liquids can effectively improve the bioresolution of ethyl 2,2-dimethylcyclopropanecarboxylate catalyzed by lipase Novozym 435. A preferred hydrophilic ionic liquid is 1-butyl-3-methylimidazolium triflate ([Bmim]OTF).
Embodiment 8~15
[0034] With reference to the method of Example 1, change the volume fraction of [Bmim]OTF in the reaction system, investigate the impact of the volume fraction of [Bmim]OTF on 2,2-dimethylcyclopropane ethyl formate bioresolution, the results are shown in the table 2:
[0035] Table 2
[0036]
[0037] Conclusion: The volume fraction of [Bmim]OTF is better in the range of 1-10%, and the optimal volume fraction is 6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com