Vitamin E succinate-chitosan graft and preparation method and application thereof
A technology of succinate and vitamin, applied in the application of stable drug nanosuspension, vitamin E succinate-chitosan graft and its preparation, new surfactant and its preparation field, can solve the problem of easy Aggregation or crystallization growth, poor physical stability of nanocrystal suspensions, affecting the stability of preparations and drug efficacy, etc., to achieve the effect of facilitating transportation and storage, improving safety and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
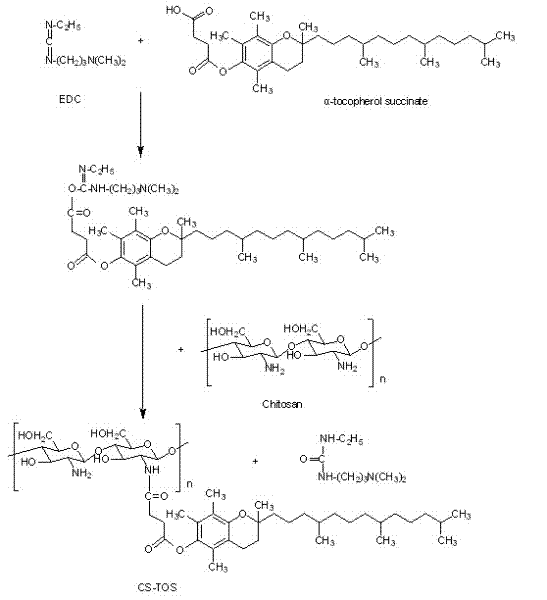
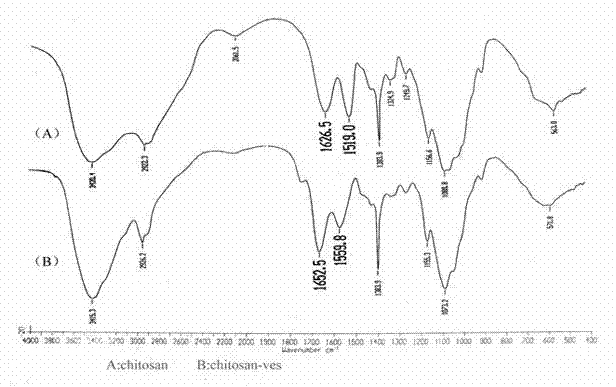
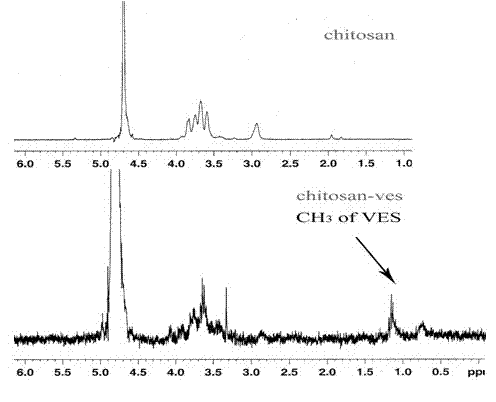
[0042] Weigh 0.2 g of chitosan (95% degree of deacetylation) with a molecular weight of 34 kDa, 0.25 g of carbodiimide and 0.15 g of N-hydroxysuccinimide to prepare an aqueous solution, and weigh 0.3 g of vitamin E succinate to dissolve In 30 ml of N,N-dimethylformamide. Under the condition of stirring, the aqueous solution containing chitosan was slowly added to the N,N-dimethylformamide solution of vitamin E succinate within 3 hours. React at 30°C for 48 hours, pour a mixed solution of 270ml of methanol and 30ml of ammonia water (9:1) into the final reaction solution, filter the precipitate to obtain a yellow gel, wash it repeatedly with absolute ethanol, and place it in a dialysis bag ( MWCO3500), distilled water dialysis for 24 hours to remove residual carbodiimide, N-hydroxysuccinimide and reaction by-products. The dialysate was freeze-dried to obtain a vitamin E succinate-chitosan graft, and the graft ratio (amino substitution degree) was determined to be 20%.
Embodiment 2
[0044] Weigh 0.2 g of chitosan (90% degree of deacetylation) with a molecular weight of 34 kDa, 0.4 g of carbodiimide and 0.4 g of N-hydroxysuccinimide to prepare an aqueous solution, and weigh 0.2 g of vitamin E succinate to dissolve In 40 ml of N,N-dimethylformamide. Under the condition of stirring, the aqueous solution containing chitosan was slowly added to the N,N-dimethylformamide solution of vitamin E succinate within 3 hours. React at 30°C for 24 hours, pour a mixed solution of 300ml of methanol and 20ml of ammonia water (15:1) into the final reaction solution, filter the precipitate to obtain a yellow gel, wash it repeatedly with absolute ethanol, and place it in a dialysis bag ( MWCO7000), distilled water dialysis for 48 hours to remove residual carbodiimide, N-hydroxysuccinimide and reaction by-products. The dialysate is freeze-dried to obtain the vitamin E succinate-chitosan graft. After determination, its grafting rate is 14%.
Embodiment 3
[0046] Weigh 1 g of chitosan (deacetylation degree 97%) with a molecular weight of 100 kDa, 0.2 g of carbodiimide and 0.2 g of N-hydroxysuccinimide to make an aqueous solution, and weigh 0.5 g of vitamin E succinate to dissolve In 30 ml of N,N-dimethylformamide. Under the condition of stirring, the aqueous solution containing chitosan was slowly added to the N,N-dimethylformamide solution of vitamin E succinate within 3 hours. React at 40°C for 48 hours, pour a mixed solution of 180ml methanol and 120ml ammonia water (6:4) into the final reaction solution, filter the precipitate to obtain a yellow gel, wash it repeatedly with absolute ethanol, and place it in a dialysis bag ( MWCO7000), distilled water dialysis for 48 hours to remove residual carbodiimide, N-hydroxysuccinimide and reaction by-products. The dialysate is freeze-dried to obtain the vitamin E succinate-chitosan graft. After determination, its grafting rate is 8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com