Method for performing fusion expression on protein containing disulfide bond
A fusion expression and protein technology, applied in the field of bioengineering, can solve problems such as inability to fold natural structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
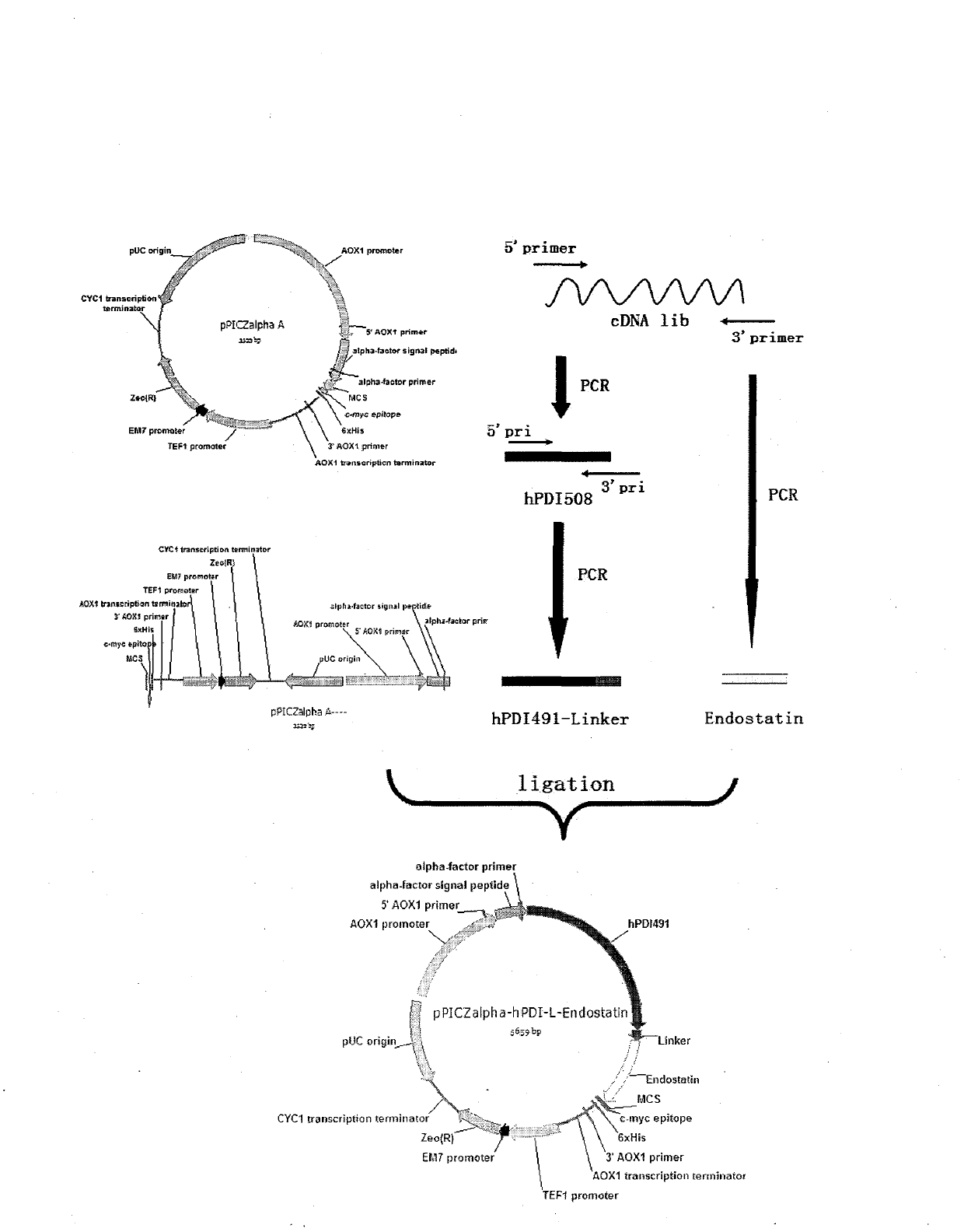
[0057] Example 1: Design and construction of recombinant plasmids and expression of fusion proteins
[0058] (1) Clone hPDI 508 cDNA gene
[0059] Referring to the hPDI gene sequence, primers were designed, and the full-length hPDI gene was amplified from a commercial human liver cDNA library by PCR. The amplified product was cut out from the restriction endonuclease Xho I and NotI sites, and recombined with the expression plasmid pPICZα of Pichia pastoris to construct the expression plasmid pPICZα-hPDI 508 , Transform NovaBlue host bacteria. Extract the plasmid, and then analyze the corresponding restriction endonuclease sites to screen the plasmid with characteristic fragments, and then use nucleotide sequence analysis to confirm the correct position and sequence of gene recombination, and obtain the hPDI full-length gene. Positive clone.
[0060] The restriction endonuclease used in the present invention comes from Takara Company, the commercialized human liver cDNA lib...
Embodiment 2
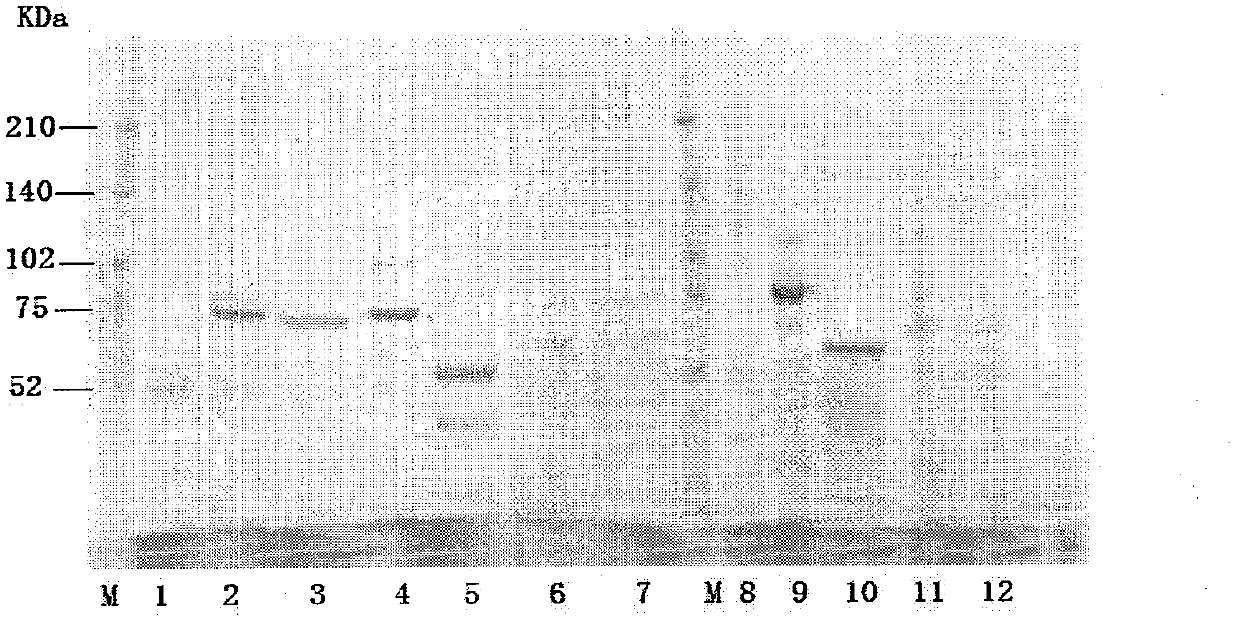
[0070] Embodiment 2: the purification of fusion protein
[0071] 1. CM Sepharose Fast Flow column chromatography
[0072] Equilibrate the column with 50mM HAc-NaAc (pH 4.2), dilute the sample solution containing the target protein until the conductance is consistent with the equilibrium buffer, adjust the pH to 4.2, start loading the sample, and wash 6 columns with the equilibrium buffer after loading volume, and then eluted with 50mM HAc-NaAc (pH 4.0) + 1M NaCl, collecting 2 column volumes.
[0073] 2.Chelating Sepharose Fast Flow Chromatography
[0074] Dilute the CM eluent to 1 time with water, then adjust the pH to 7.4 with sodium hydroxide, put it on the Chelating Sepharose Fast Flow column that has been equilibrated with 50mM Tris-HCl (pH 7.4)+0.5M NaCl, and use the equilibration buffer after loading Rinse 6 column volumes, then wash away impurities with 50mM Tris-HCl (pH 7.4) + 20mM imidazole, and finally elute with 50mM Tris-HCl (pH 7.4) + 200mM imidazole, and collec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com