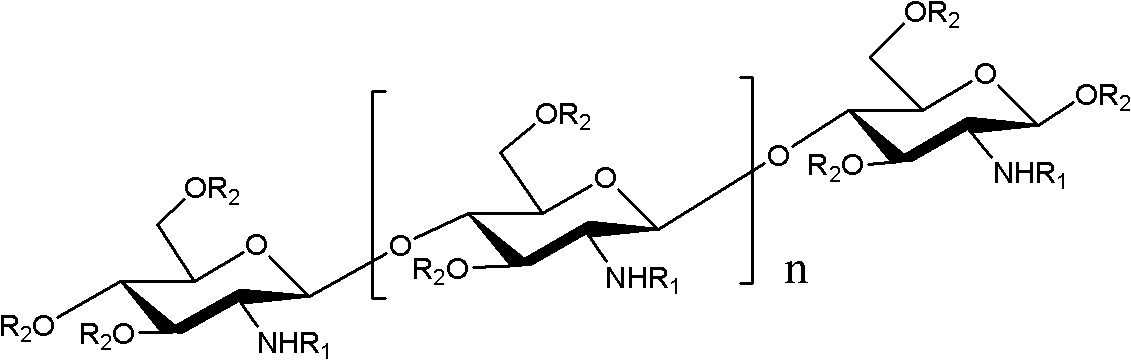
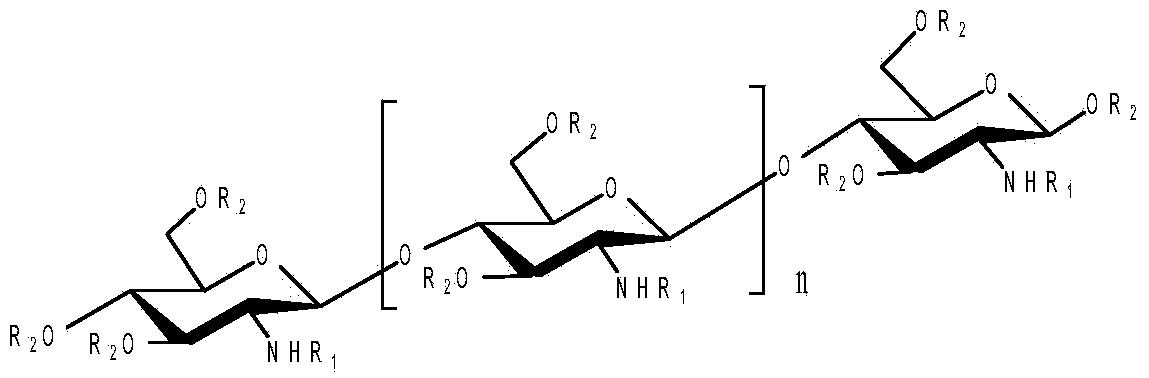
O-unsaturated fatty acid acylated chitosan oligosaccharides as well as preparation and application thereof
A technology for fatty acid acylation of chitosan oligosaccharide and unsaturated fatty acid, which is applied in the field of O-unsaturated fatty acid acylated chitosan oligosaccharide and its preparation, and can solve problems such as insufficient activity, insufficient stability of unsaturated fatty acid, and troublesome use and storage. , to achieve the effect of high selectivity, clear structure and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of O-linoleic acid acylated chitosan oligosaccharide
[0031] Get 1.62g of chitosan oligosaccharide with a degree of polymerization of 2-8 and dissolve it in 30mL of N,N-dimethylformamide, add 1.63g of p-methoxybenzaldehyde, stir at room temperature for 4 hours, add 5 Doubling the volume of ethanol, a large amount of light yellow precipitates were produced, suction filtered, the filter cake was washed 3 times with anhydrous acetone, and vacuum-dried to obtain 2.5 g of light yellow powder, which was amino schiff base protected chitosan oligosaccharide.
[0032] Get 2.8g amino-protected oligochitosan and dissolve in N, N-dimethylformamide, N, N-dimethylformamide; Add 0.1g dimethylaminopyridine; Add 3.0g linoleic acid N , N-dimethylformamide, react the above solution at 60°C for 6 hours, add ethanol 4 times its volume to the reaction solution to produce a large amount of light yellow precipitate, filter it with suction, and wash the filter cak...
Embodiment 2
[0036] Embodiment 2: the preparation of O-linolenic acid acylated chitosan oligosaccharide
[0037] Take 1.62g of chitosan oligosaccharide with a degree of polymerization of 2-15 and dissolve it in 30mL of N,N-dimethylformamide, add 1.63g of benzaldehyde, stir at room temperature for 4 hours, add 5 times its volume of ethanol to the reaction solution , produced a large amount of light yellow precipitate, suction filtered, the filter cake was washed 3 times with anhydrous acetone, and vacuum dried to obtain 2.5 g of light yellow powder, which was amino schiff base protected chitosan oligosaccharide.
[0038] Get 2.8g amino-protected oligochitosan and dissolve in N, N-dimethylformamide, N, N-dimethylformamide; Add 0.1g dimethylaminopyridine; After adding 4.1g linolenic acid N, N-dimethylformamide, react the above solution at 60°C for 8 hours, add ethanol 4 times its volume to the reaction solution to produce a large amount of light yellow precipitate, filter with suction, wash t...
Embodiment 3
[0042] Example 3: myristoleic acid, palmitoleic acid, oleic acid, octadecatrienoic acid, eicosapentaenoic acid, docosahexaenoic acid, octadecadienoic acid, octadecatriene Preparation of O-acylated chitosan oligosaccharides such as acid and eicosatetraenoic acid
[0043] According to the method of embodiment 1 and 2, unsaturated fatty acid raw material is selected from myristoleic acid, palmitoleic acid, oleic acid, octadecatrienoic acid, eicosapentaenoic acid, docosahexaenoic acid, eicosapentaenoic acid, Octadecadienoic acid, octadecatrienoic acid, eicosatetraenoic acid, O-myristoleic acid acylated chitosan oligosaccharide, O-palmitoleic acid acylated chitosan oligosaccharide, O-oleic acid can be prepared Acylated oligochitosan, O-octadecatrienoic acid acylated oligochitosan, O-eicosapentaenoic acid acylated oligochitosan, O-docosahexaenoic acid acylated oligochitosan, O - Octadecadienoic acid acylated oligochitosan, O-octadecadienoic acid acylated oligochitosan, O-eicosatetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com