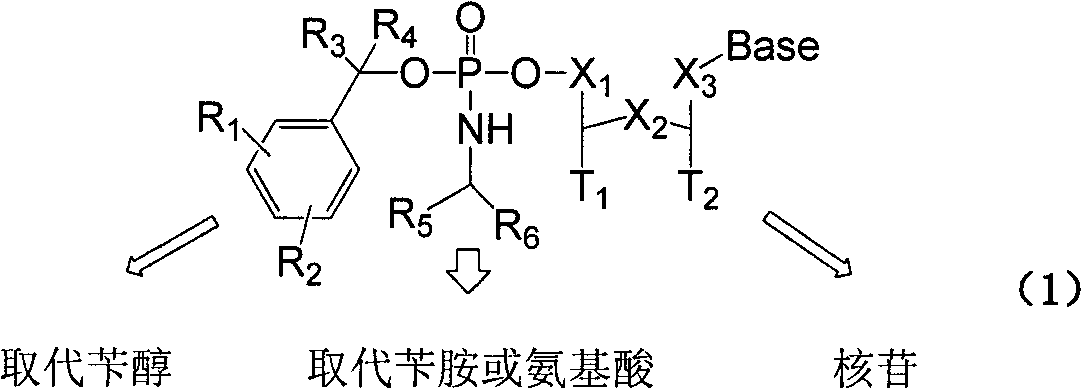
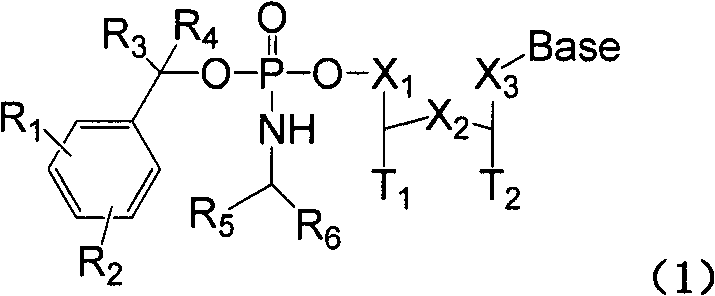
Structure and synthesis of novel benzyl amido phosphate prodrug of nucleoside compound
A technology of benzyl phosphoramidate and compounds, applied in the fields of compounds of elements of group 5/15 of the periodic table, organic chemistry, antineoplastic drugs, etc., which can solve the problems of carcinogenic toxicity and lower bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] A solution of 30 mmoles of appropriately substituted benzyl alcohol and triethylamine (4.25 mL, 30.5 mmol) in dry benzene (125 mL) was slowly added dropwise to freshly distilled phosphorus oxychloride (10 mL, 107 mmol) at 0-10 °C In dry benzene (50 mL), the reaction solution was slowly warmed up and stirred at room temperature overnight. The reaction mixture was filtered under dry conditions, and the filtrate was concentrated to give substituted benzyl phosphoryl dichloride (12).
Embodiment 2
[0065]
[0066] Dissolve substituted benzylphosphoryl dichloride (12) (4mmol) and appropriately substituted benzylamine (13, 4mmol) into dry dichloromethane (50mL), slowly add three Ethylamine (0.6 mL, 4.3 mmol) in dichloromethane (10 mL). The reaction solution was stirred and reacted at room temperature for 5 hours, the solvent was evaporated, dry diethyl ether (25 mL) was added to the residue, filtered under nitrogen protection, and the N, O-disubstituted benzylphosphoryl chloride (14) obtained after the filtrate was concentrated could be directly For the next reaction without purification.
[0067] The obtained N,O-disubstituted benzyl phosphoryl chloride (14) can also be dissolved in dry tetrahydrofuran (4 mL) to prepare a 1 mol / L solution for use.
Embodiment 3
[0069]
[0070] At room temperature, N-methylimidazole (410 mg, 5 mmol) was slowly added dropwise to a suspension of uracil (244 mg, 1 mmol) in anhydrous THF (5 mL), and stirred at room temperature for 30 minutes. Then, a solution of N, O-dibenzylphosphoryl chloride (14b, 618 mg, 2 mmol) in THF (2 mL) was slowly added to the above reaction mixture, and the reaction was stirred overnight at room temperature. The reaction solution was concentrated by rotary evaporation, the residue was dissolved in chloroform (10 mL), the organic phase was washed with dilute hydrochloric acid (1M, 10 mL), saturated sodium bicarbonate (10 mL), water (15 mL), and dried (Na 2 SO 4 ), concentrated, and the synthesized uracil benzyl phosphoramidate was purified by silica gel column chromatography (dichloromethane:methanol=10:1). MS(m / e)518(M+H). 1 H NMR (CD 3 OD, 400MHz) δ7.66(d, J=6.8Hz, 0.5H), 7.58(d, J=8.4Hz, 0.5H), 6.98-7.32(m, 9H), 5.72(d, J=5.6Hz, 0.5H), 5.70(d, J=5.2Hz, 0.5H), 5.47(, d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com