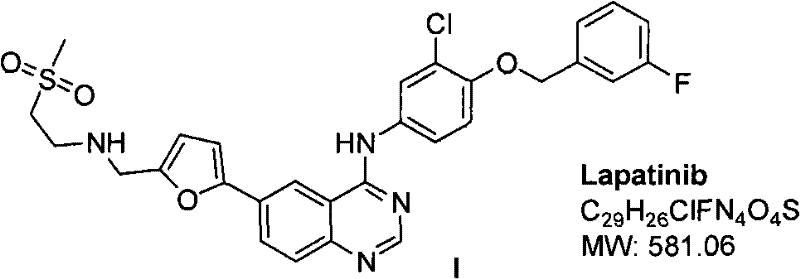
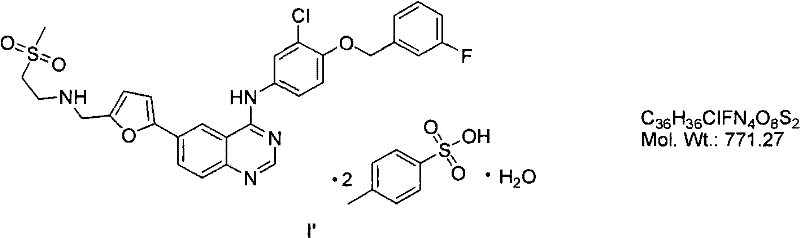
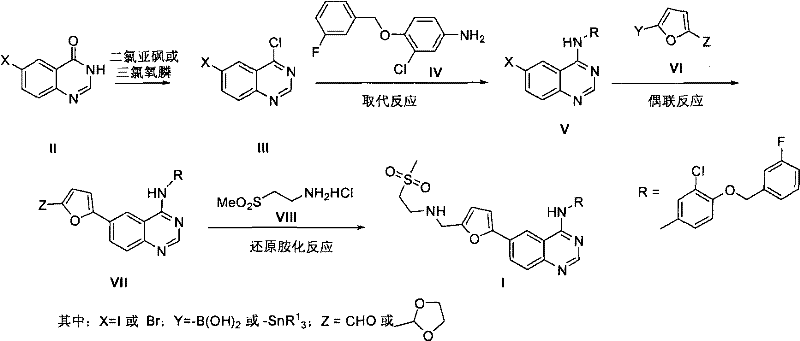
Synthetic method of lapatinib and salt of lapatinib
A technology of lapatinib and a synthesis method is applied in the field of preparation of related intermediates, and can solve the problems of high cost of raw materials, difficulty in controlling by-products, troublesome product purification and post-processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: N-[3-chloro-4-[(3-fluorobenzyl)oxy]phenyl]-6-bromo-quinazolin-4-amine (V-Br)
[0074]
[0075] In a 100mL round bottom flask equipped with a nitrogen gas guide tube, a reflux condenser (with a drying tube at the end), a mechanical stirrer and a thermometer, add 2-amino-5-bromoxynil (3.9g), toluene (30mL), N , N-dimethylformamide formal (DMF-DMA) (6.3mL) and anhydrous acetic acid (0.06mL), under stirring, heated to reflux temperature, reacted for about 8 hours, and TLC detected that the raw materials disappeared. After cooling, the solvent was evaporated under reduced pressure. Under nitrogen protection, 3-chloro-4-(3-fluorobenzyloxy)aniline (5.0 g) and anhydrous acetic acid (30 mL) were added to the residue, and the reaction was refluxed for 10 hours. TLC detected that the raw material basically disappeared, and cooled; after recovering acetic acid under reduced pressure, the obtained residue was slowly poured into saturated aqueous sodium carbonate solut...
Embodiment 2
[0078] Example 2: N, N-dimethyl-N'-(2-cyano-4-bromophenyl)-formamidine (X-Br)
[0079]
[0080] In a 100mL round bottom flask equipped with a nitrogen gas guide tube, a reflux condenser (with a drying tube at the end), a mechanical stirrer and a thermometer, add 2-amino-5-bromoxynil (3.9g), xylene (30mL) successively, N,N-Dimethylformamide formal DMF-DMA (6.3mL) and anhydrous acetic acid (0.06mL) were stirred, heated to reflux temperature, reacted for about 5 hours, TLC detected that the raw materials disappeared. After cooling, the solvent was evaporated under reduced pressure. The residue was purified by column chromatography to obtain 4.5 g of N,N-dimethyl-N'-(2-cyano-4-bromophenyl)-formamidine, calculated as 2-amino-5-bromoxynil, Yield 89.4%.
[0081] Melting point: 48-49°C; ESI-MS: m / z 252 / 254 (M+H) +
[0082] 1H NMR (400MHz, CDCl 3 )δ: 7.62(d, J=2.4Hz, 1H), 7.58(s, 1H), 7.49(dd, J=2.0, 8.8Hz, 1H), 6.83(d, J=8.4Hz, 1H), 3.09( s, 3H), 3.08(s, 3H) ppm.
Embodiment 3
[0083] Embodiment 3: N, N-dimethyl-N'-(2-cyano-4-iodophenyl)-formamidine (X-I)
[0084]
[0085]In a 250mL round bottom flask equipped with a nitrogen gas guide tube, a reflux condenser (with a drying tube at the end), a mechanical stirrer and a thermometer, add 2-amino-5-iodobenzonitrile (3.9g), toluene (50mL), N , N-dimethylformamide formal DMF-DMA (6.3mL) and anhydrous acetic acid (0.02mL), under stirring, heated to reflux temperature, reacted for about 7 hours, and TLC detected that the raw materials disappeared. Cool and concentrate under reduced pressure to obtain an oil. After curing at low temperature, wash with anhydrous ether to obtain 4.5 g of N,N-dimethyl-N'-(2-cyano-4-iodophenyl)-formamidine, which was mixed with 2-amino-5-iodobenzene Based on nitrile calculation, the yield was 75.3%.
[0086] ESI-MS m / z 300.1(M+H) +
[0087] 1H NMR (400MHz, CDCl 3 )δ: 7.79(d, J=1.9Hz, 1H), 7.65(dd, J=1.9, 8.5Hz, 1H), 7.57(s, 1H), 6.70(d, J=8.2Hz, 1H), 3.08( s, 6H) ppm. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com