Catalyst for synthesizing dimethyl carbonate and preparation method thereof
A technology of dimethyl carbonate and catalyst, which is applied in the field of catalyst and its preparation for oxidative carbonylation of methanol to dimethyl carbonate, can solve the problems of low DMC yield and low conversion rate of methanol, and achieve high DMC yield and methanol High conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Catalyst composition, preparation method and activity evaluation of the present invention
[0042] 1. Catalyst composition:
[0043] Active ingredient: CuO-CoO
[0044] Cocatalyst: K 2 o
[0045] Carrier: ZSM-5 (5A-molecular sieve)
[0046] 2. Preparation method:
[0047] (1) Prepare 100 mL each of 3 mol / L copper nitrate, cobalt nitrate and potassium nitrate respectively, and set aside.
[0048] (2) According to Cu(II): Co(II): K(I) is the proportion shown in Table 1 (the proportion shown in Table 1 is the ratio of moles, and the catalyst composition is selected by orthogonal test method) to take the above Put the stock solution into a 100mL beaker and mix it for later use.
[0049] (3) Weigh 10g ZSM-5 (among them, SiO 2 : Al 2 o 3 ≈2) into the mixture of (2).
[0050] (4) Put the beaker into an ultrasonic oscillator, set the temperature at 20° C., and the oscillation frequency at 60 Hz, and disperse for 6 hours. The purpose of dispersion is to m...
Embodiment 2
[0060] Embodiment 2: Catalyst preparation condition optimization experiment
[0061] Weigh K with a Cu / Co / K molar ratio of 1.00:0.75:0.03 2 1.0g of O-Cu-Co / ZSM-5 composite catalyst was loaded into a pressurized continuous fixed-bed differential reactor, and hydrogen gas was introduced at a flow rate of 5.0ml / min. The reduction temperature was 280°C, the reduction time was 2 hours, and the reduction pressure was 2MPa. Stop feeding hydrogen, switch to methanol, CO, O 2 , to evaluate the activity of the catalysts prepared under different conditions, the results are shown in Table 2-8.
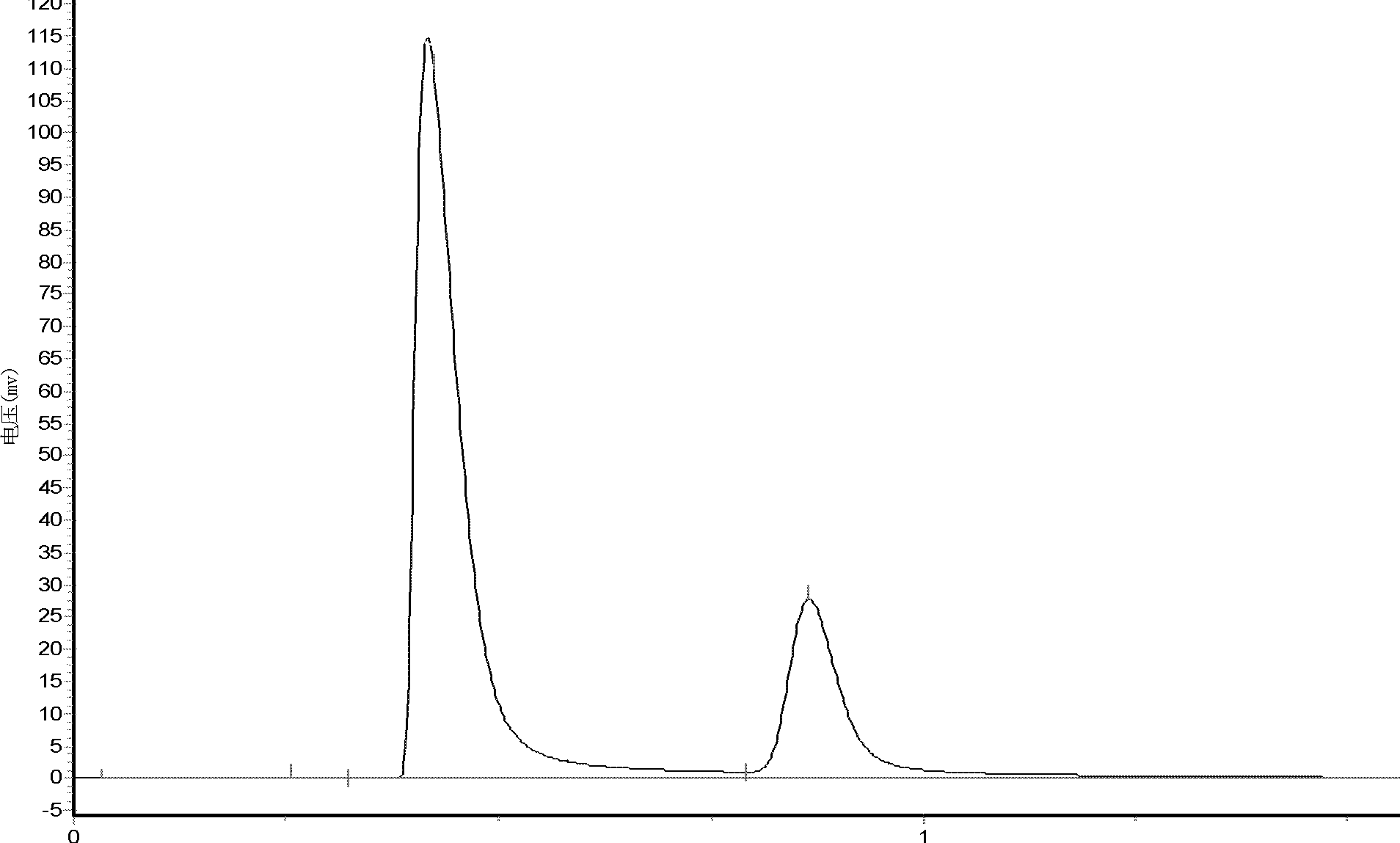
[0062] The activity was evaluated using GC112A gas chromatograph and thermal conductivity cell detector. The conditions are: chromatographic column: GDX-502; detector: TCD; carrier gas: H 2 ;Carrier gas flow rate: 5.0mL min -1 ; Injector temperature: 150°C; Detector temperature: 150°C; Column temperature: 130°C. CO conversion and DMC (dimethyl carbonate) yield were calculated using the area...
Embodiment 3
[0092] Example 3 Catalyst Performance Evaluation Experiment
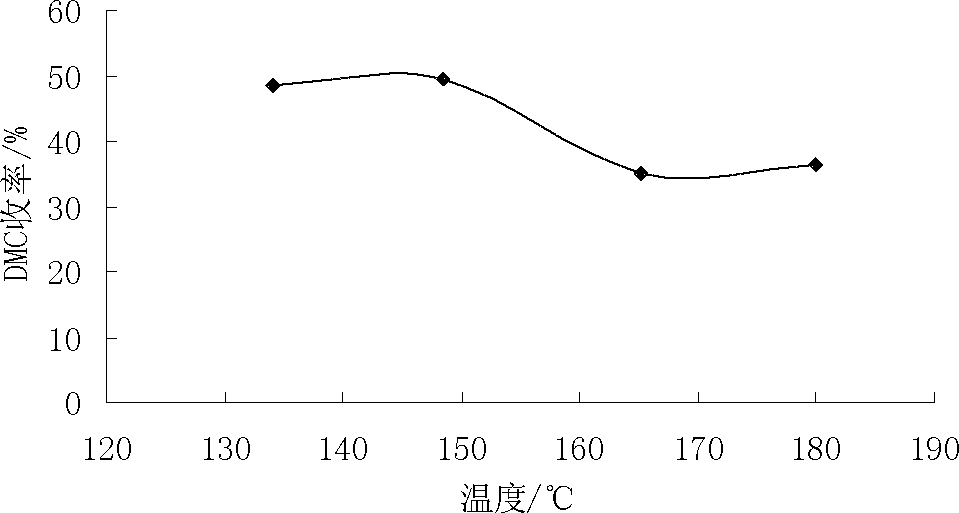
[0093] This example evaluates the influence of reaction time, reaction temperature, reaction pressure, and raw material ratio on the performance of the catalyst of the present invention, wherein the catalyst prepared in Example 1 above with K / Cu / Co=0.03:1.00:0.75 is taken as an example.
[0094] The catalyst of this embodiment is applied in the oxidative carbonylation synthesis of dimethyl carbonate with methanol, carbon monoxide and oxygen as raw materials, and the main reaction chemical formula is as follows:
[0095] 2CH 3 OH+CO+1 / 2O 2 →(CH 3 O) 2 CO+H 2 o
[0096] Methanol flow rate 0.21ml / min.
[0097] The specific performance evaluation experiments are as follows:
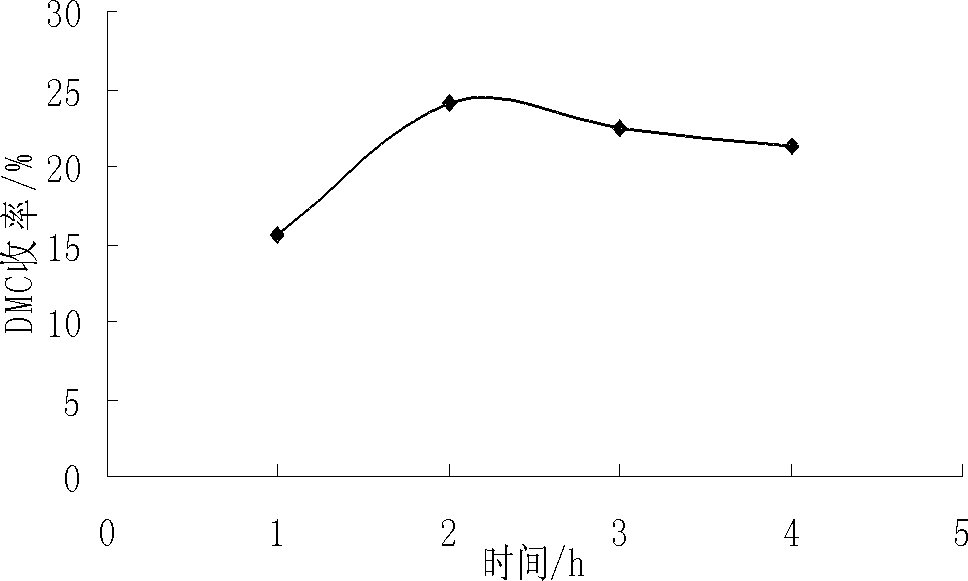
[0098] 1. Effect of reaction time on catalyst performance
[0099] When the reaction temperature is 165℃, CO / O 2 =10:1 (ratio of moles), when the reaction pressure is 0.2Mpa, the influence of different reaction times on the synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com