Preparation method of dezocine
A technology for dezocine and intermediates, which is applied in the field of preparation of dezocine, can solve the problems of low splitting efficiency and unsatisfactory splitting efficiency, and achieves cheap and easy-to-obtain reagents, mild reaction conditions, and simple and efficient process operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
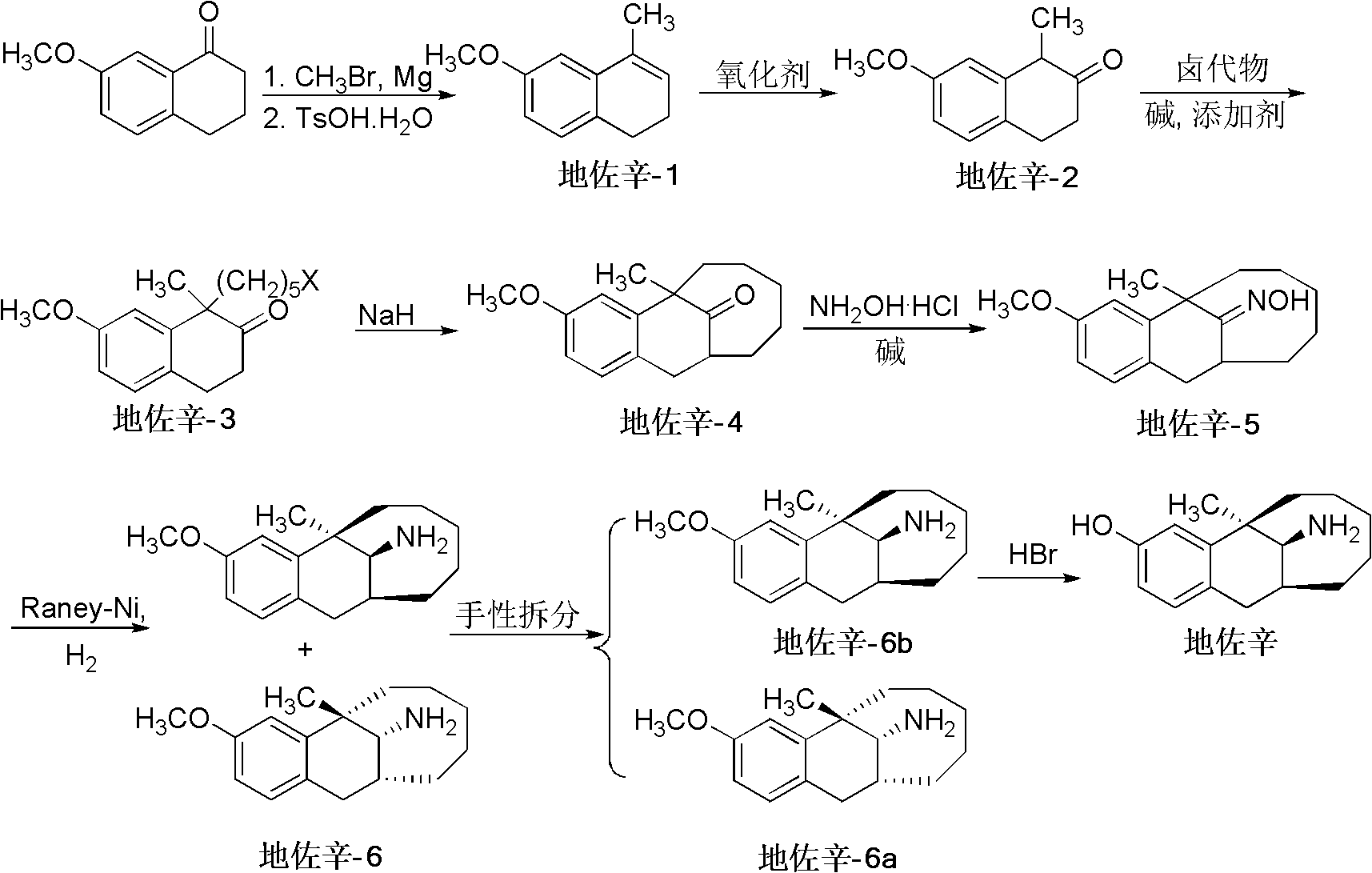
[0042] Embodiment 1 prepares dezocine
[0043] The preparation method of the dezocine of the present embodiment comprises the following steps:
[0044] 1) Synthetic intermediate "Dezocine-1", raw material ratio:
[0045] raw material name
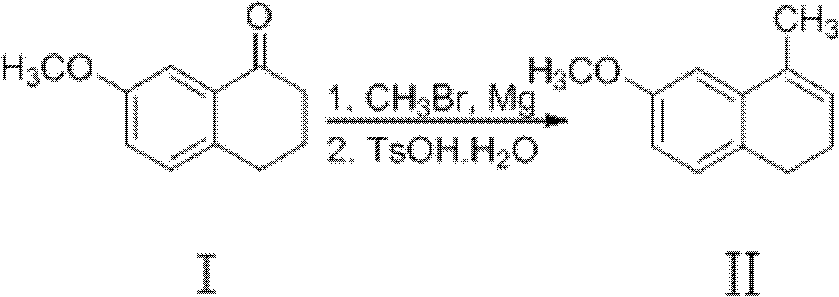
[0046] Reaction steps: under a nitrogen atmosphere, add magnesium chips (5kg, 210mol) and anhydrous ether (90L) into a dry reactor, use an ice-water bath to cool down, and slowly add bromomethane (23.5kg, 250mol) dropwise while stirring Diethyl ether (20L) solution, keep diethyl ether slightly reflux state. After dropping, heat to reflux and react for 1 hour to dissolve the magnesium bars completely. The starting material 7-methoxy-3,4-dihydro-1-naphthalenone (22.6kg, 130mol) in toluene (60L) was slowly dropped into the above-mentioned Grignard reagent under stirring, and the temperature was kept at 28 ~ 32°C, keep the original temperature after dropping and continue the reaction for 1h. Cool in an ice-salt bath, slowly add s...
Embodiment 2
[0077] Embodiment 2 prepares dezocine
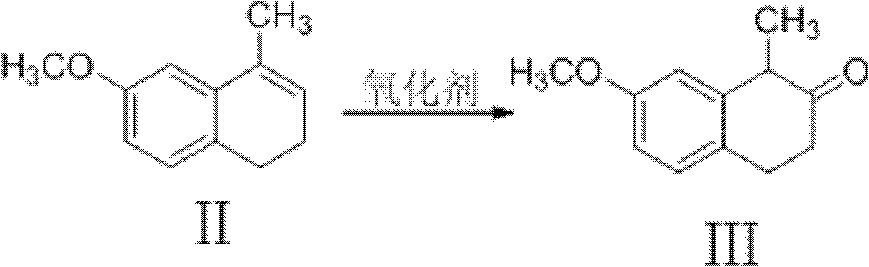
[0078] The intermediate "Dezocine-1" was first synthesized according to the method in Example 1, and then the intermediate "Dezocine-2" was prepared from the intermediate "Dezocine-1" according to the following reaction steps.
[0079] The intermediate "Dezocine-1" (18.84kg, 110mol) was dissolved in chloroform (25L), and a solution of peroxyphenylacetic acid (20kg, 132mol) in chloroform (15L) was added dropwise at 0°C while stirring. After dropping, continue to stir for 4 hours at 0-5°C. After the reaction of the raw materials is monitored by TCL, 10% sodium hydroxide solution (30L) is added to the reactor, stirred for a while and left to separate liquids, and the organic phase is separated with 10% sodium hydroxide Wash with NaOH solution (2×10L), combine the aqueous phases and extract with chloroform (2×10L), combine the organic phases, wash with water (10L) once, and evaporate the solvent to obtain an oily substance. A mixture of 95%...
Embodiment 3
[0081] Embodiment 3 prepares dezocine
[0082] The intermediate "Dezocine-1" was first synthesized according to the method in Example 1, and then the intermediate "Dezocine-2" was prepared from the intermediate "Dezocine-1" according to the following reaction steps.
[0083] The intermediate "Dezocine-1" (18.84kg, 110mol) was dissolved in chloroform (25L), and a solution of peracetic acid (10kg, 132mol) in chloroform (15L) was added dropwise at 0°C while stirring. After dropping, continue to stir for 4 hours at 0-5°C. After the reaction of the raw materials is monitored by TCL, 10% sodium hydroxide solution (30L) is added to the reactor, stirred for a while and left to separate liquids, and the organic phase is separated with 10% sodium hydroxide Wash with NaOH solution (2×10L), combine the aqueous phases and extract with chloroform (2×10L), combine the organic phases, wash with water (10L) once, and evaporate the solvent to obtain an oily substance. A mixture of 95% ethanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com