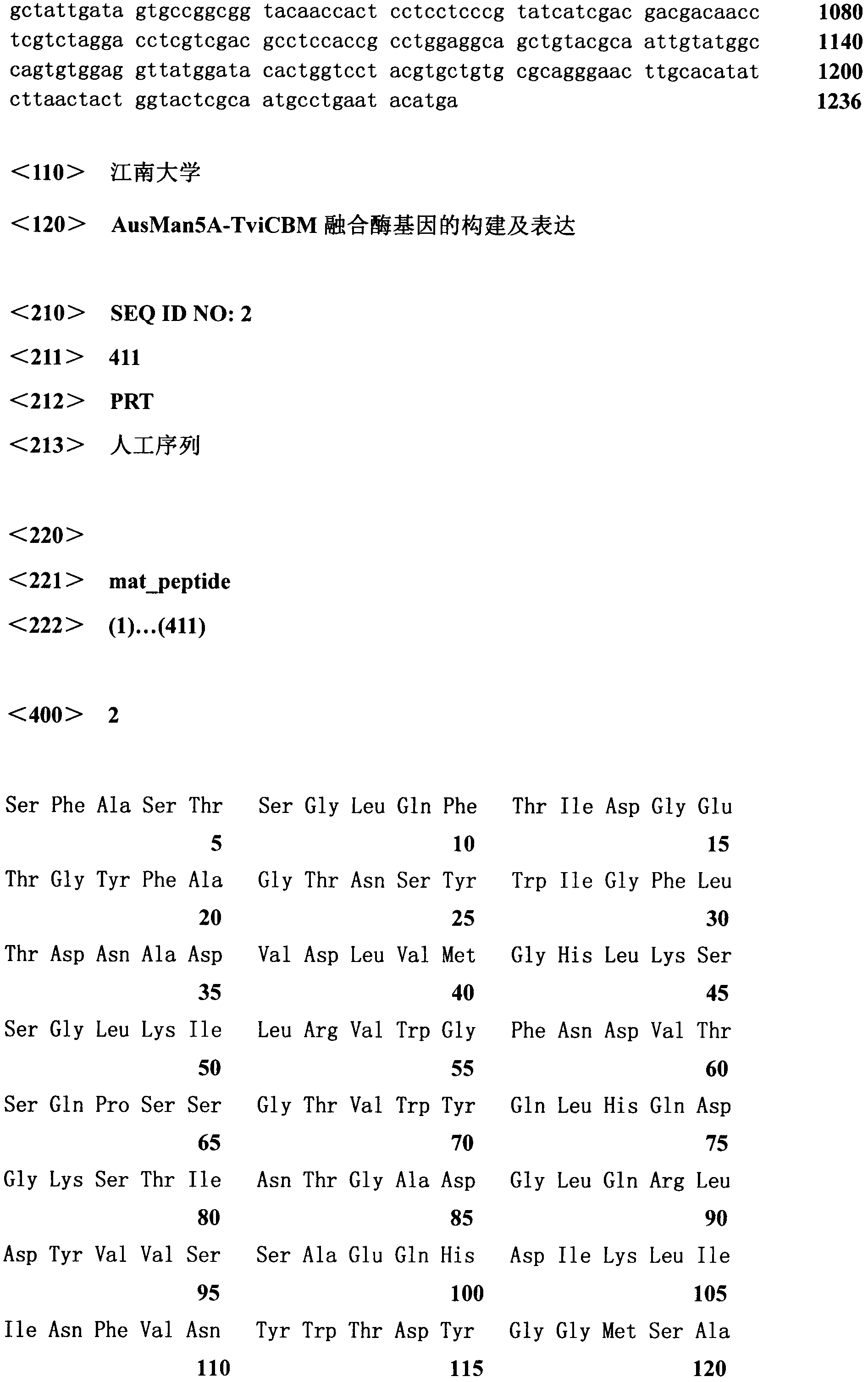Construction and expression of AusMan5A-TviCBM fusion enzyme gene
A technology for fusing enzymes and gene fragments, which is applied in the fields of genetic engineering and protein expression, can solve the problems of unreported research on design and expression, and achieve the effects of large-scale industrial production, high catalytic efficiency, and strong substrate affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Construction of chimeric enzyme gene and its expression plasmid
[0027]The fusion gene man5A-cbm was constructed by overlapping PCR technology, which was mainly divided into four steps: the first round of PCR was performed with AMan5A-F and AMan5A-R as primers (94°C for 4 min; 30 cycles, 94°C for 30s, 53°C for 30s, 72 ℃1min; 72℃10min) to synthesize the gene fragment of Aspergillus ussami Aus Man5A to be fused; use Tman5A-F1 and Tman5A-R as primers for the second round of PCR (94℃4min; 30 cycles, 94℃30s, 53℃30s , 72 °C for 1 min; 72 °C for 10 min) to synthesize the gene fragment of the CBM to be fused; the two rounds of PCR products were analyzed by 1% agarose gel electrophoresis, and the target bands were recovered by tapping the gel. The third round of PCR was performed at 94°C for 4 min; 10 cycles of 94°C for 30s, 52°C for 30s, 72°C for 70s; 72°C for 10min); the third round of PCR reaction solution was used as the template, AMan5A-F and Tman5A-R were used a...
Embodiment 2
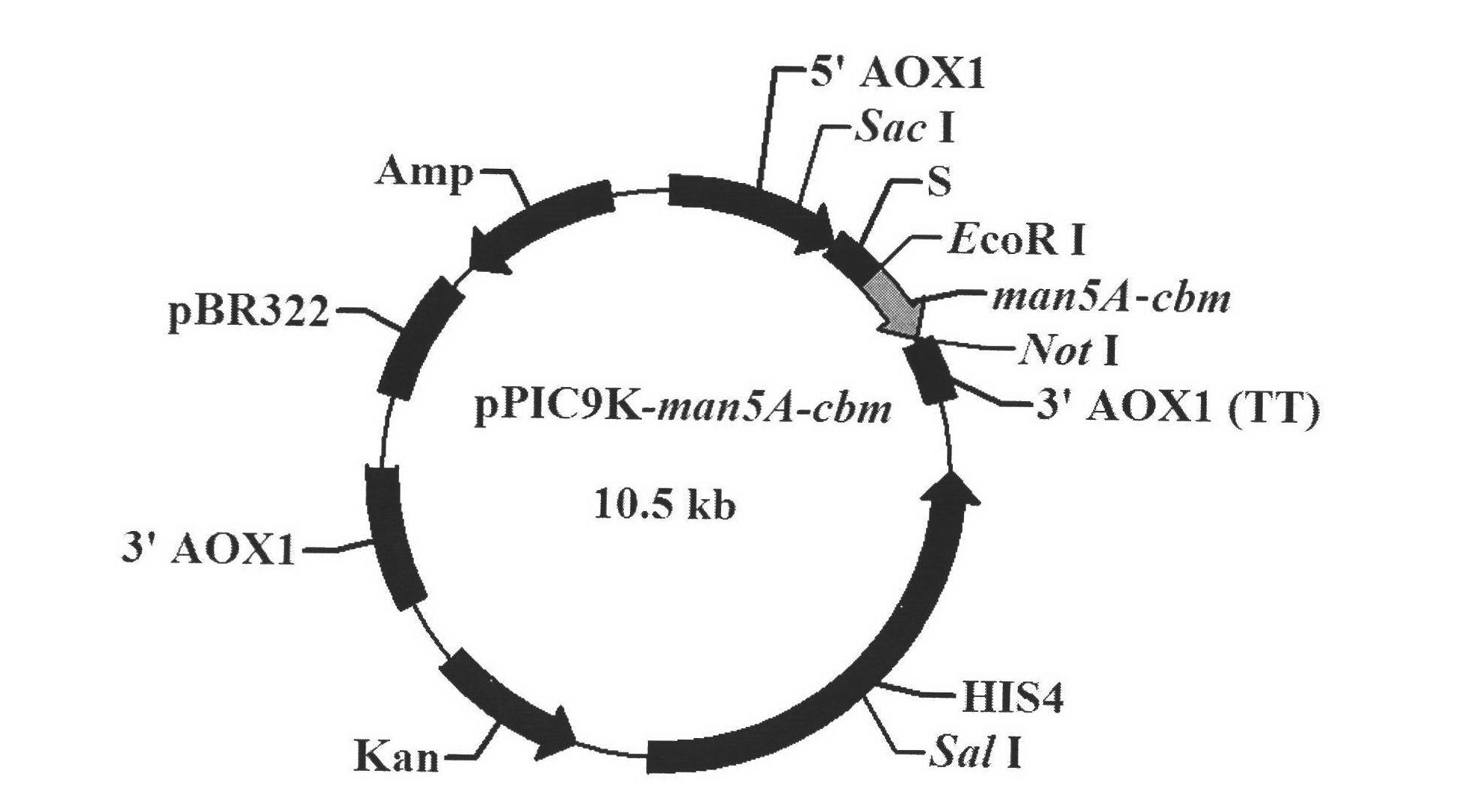
[0028] Example 2 Construction, expression, product purification and activity assay of GS115 / man5A-cbm
[0029] The pPIC9K-man5A-cbm was linearized with Sal I, and electro-transformed and screened according to the Pichia expression manual to obtain a high-copy Pichia recombinant GS115 / man5A-cbm. The genetically engineered bacteria were induced to express with 1.0% methanol for 96 hours, and the mannanase activity in the fermentation broth was measured by DNS method to reach 65 IU / mL. The supernatant of the fermentation broth after centrifugation is the crude enzyme solution of the chimeric enzyme. The crude enzyme solution is concentrated by an ultrafiltration membrane with a molecular weight cut-off of 10,000 Da, and then subjected to DEAE-Sepharose Fast Flow ion exchange chromatography and Sephadex G-75 It was purified by gel filtration chromatography and detected as a single band by SDS-PAGE after purification.
[0030]
[0031]
[0032]
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com