Application of diosgenin and derivative thereof for preparing tumor chemotherapy sensitization medicines
A technology of diosgenin and derivatives is applied in the application field of preparing tumor chemotherapy sensitizing drugs, and can solve the problem that diosgenin is not widely used and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
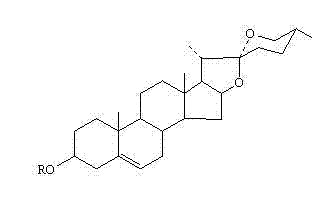
[0057] Obtaining Diosgenin and Its Derivatives
[0058] Diosgenin and its derivatives can be purchased from commercial products or prepared by the following methods:
[0059] For the preparation method of diosgenin, see patent 200610040991.1 (Tang Qinghua, Yao Yunshan, Tang Xiaohui. A method for extracting cantharidin. Intellectual Property Office of the People's Republic of China).
[0060] Diosgenin and its derivatives can be purchased from commercial products or prepared by the following methods:
[0061] For the preparation method of diosgenin, see patent 200610028164.0 (Zhu Xian, Guo Xiaoya, Wang Zhenwu. Method for preparing diosgenin by super (near) critical water hydrolysis. Intellectual Property Office of the People's Republic of China).
[0062] For the preparation method of dioscin, see patent 02146284 (Zhao Quancheng, He Yufang, Liu Wei. Preparation method of dioscin, pharmaceutical preparation and its new medical application. Intellectual Property Office of the ...
Embodiment 2
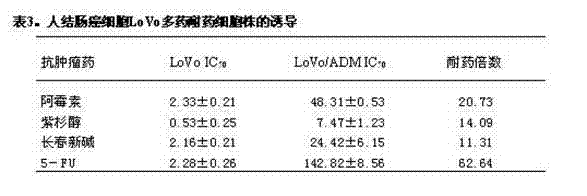
[0065] Induction of multidrug resistant cell lines
[0066] Human liver cancer cell HepG2, human leukemia cell K562, human colon cancer cell LoVo cell, human breast cancer cell MCF-7, human lung cancer cell A549, human gastric cancer cell SGC7901, ovarian cancer SKOV3, cervical cancer Hela, kidney cancer 786-0 or prostate cancer PC-3 were inoculated into 6-well plates for culture, and ADM with a final concentration of 0.05 μg / ml was added the next day, and the medium was changed every 2-3 days, and the concentration of ADM was gradually increased at the same time; After 3 times, a large number of cells will die, and a small amount of cells can still be adhered to the wall. Continue to gradually increase the concentration of ADM until the remaining adherent cells form a single cell clone; when the cells form a large clone, digest the cells with trypsin, Disperse evenly, and continue to add ADM induction until the cells can be normally passaged, frozen and recovered in ADM wit...
Embodiment 3
[0068] Identification of multidrug resistant cell lines
[0069] Sensitive cells and drug-resistant cells were inoculated in 96-well plates at a cell density of 5×10 4 a / ml;
[0070] Dosing on the next day, the concentration gradient of doxorubicin: 30, 3, 0.3, 0.03, 0.003μg / ml; the concentration gradient of vincristine: 10, 1, 0.1, 0.01, 0.001μg / ml; the concentration of paclitaxel Gradient: 10, 1, 0.1, 0.01, 0.001 μg / ml; 5-FU concentration gradient: 10, 1, 0.1, 0.01, 0.001 μg / ml, drugs were doubled with DMEM medium containing 3% FBS Ratio dilution, the negative group was added with DMEM medium containing 3% FBS, each group was set up with 3 replicate holes, and the volume of administration was 100 μl / well; the cells were treated with 5% CO 2 After continuing to culture in a 37°C incubator for 44 hours, add 20 μl MTT (5 mg / ml) to each well, and continue to culture for 4 hours; discard the supernatant, add 100 μl DMSO to each well, shake on a microplate reader for 600 s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com