Thermosetting polyphenylquinoxaline resin, its preparation method and application
A technology of polyphenylquinoxaline and polymerization reaction, which is applied in the field of high-performance thermosetting and high-temperature-resistant resins, can solve problems such as no systematic reports, and achieve the effect of excellent high-temperature resistance performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
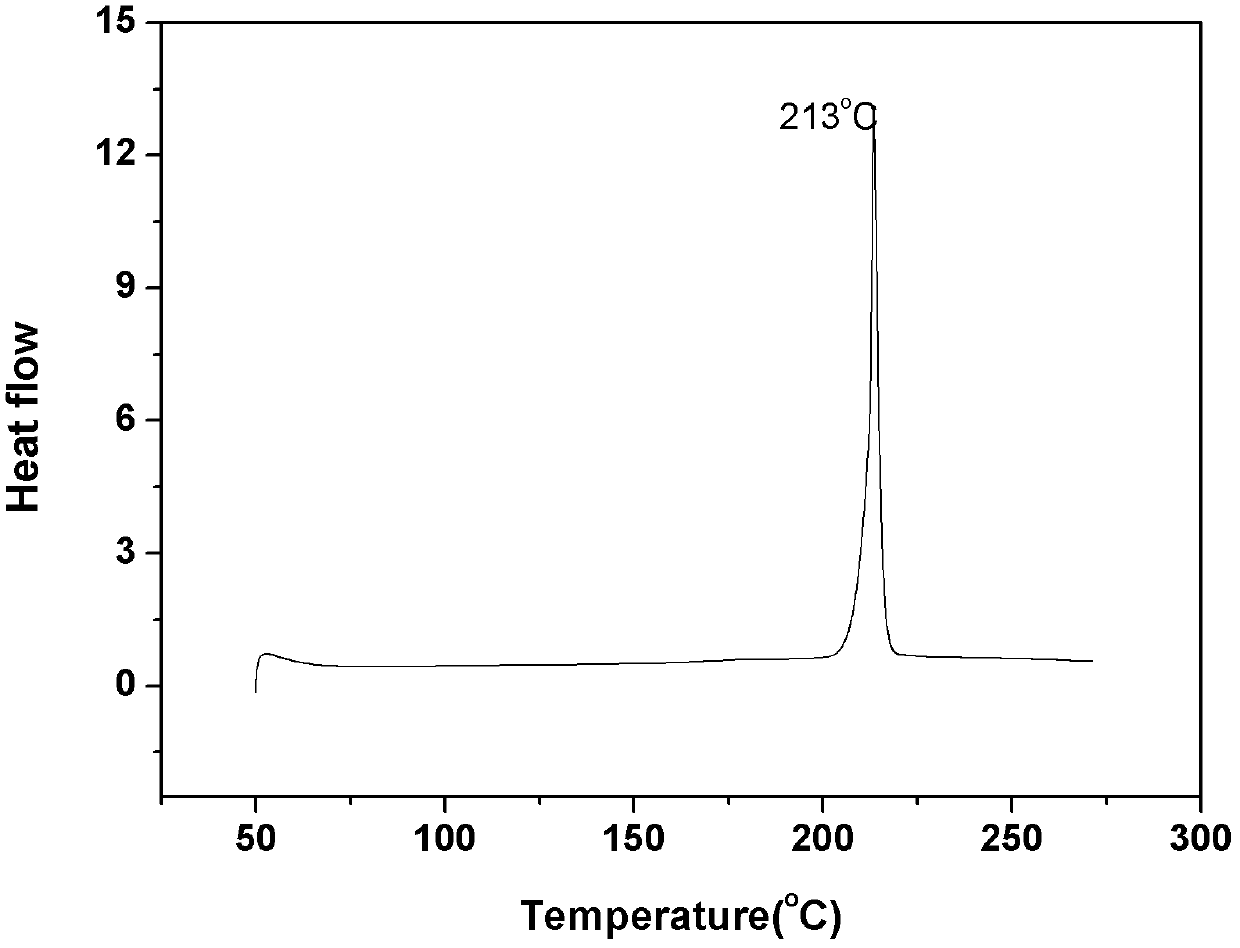
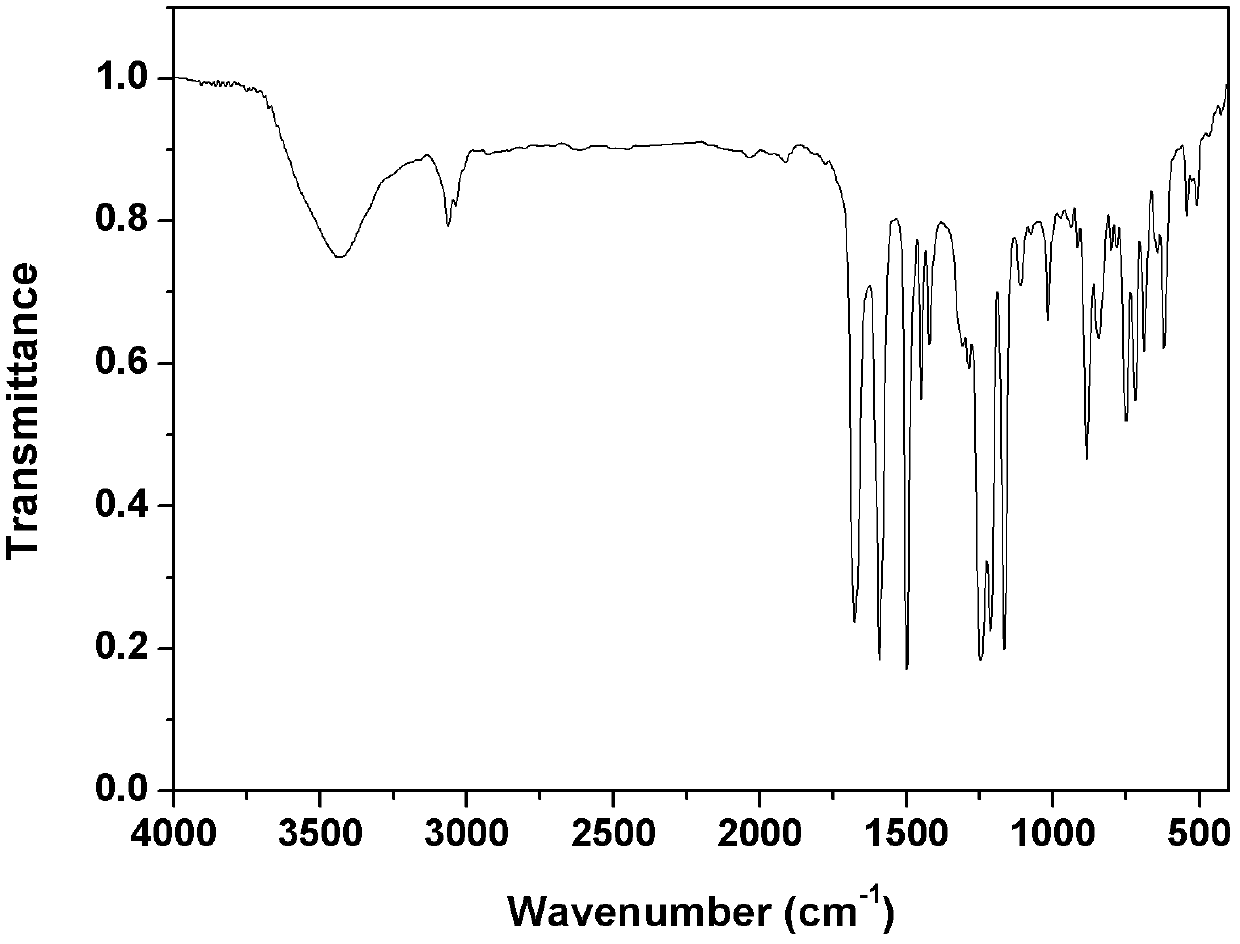
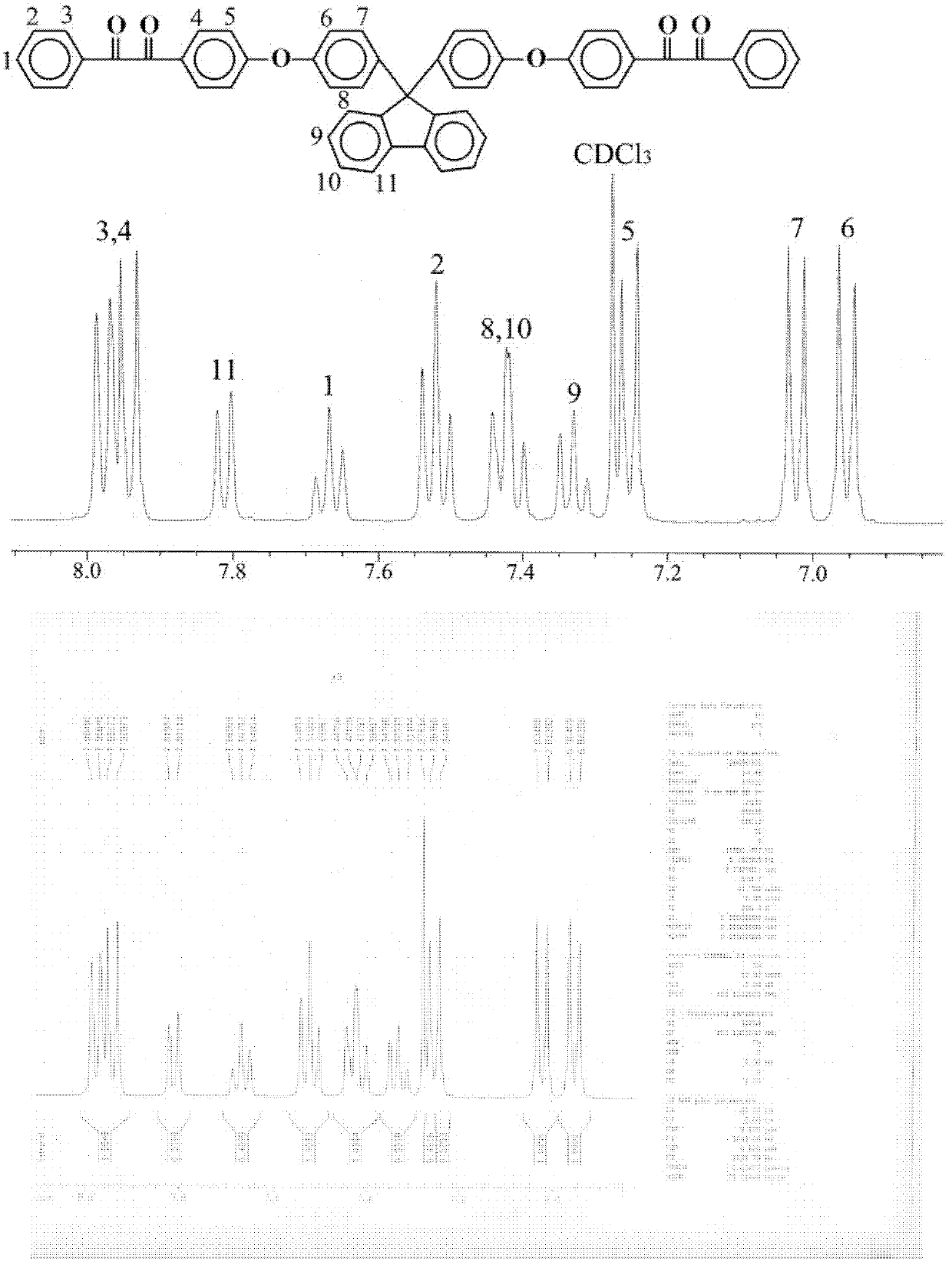
[0044] Example 1. Synthesis of phenylethynyl-terminated PPQ (in formula I, Y=-) with a designed molecular weight of 2500 g / mol
[0045] 1) Tetraketone compound 9 shown in formula II, the synthesis of 9-bis[(4-benziloxy)phenyl]fluorene
[0046] Add 53.60g (0.21mol) of 4-nitrobenzil, 35.04g (0.10mol) of 9,9-bis[(4-hydroxyphenoxy ) phenyl] fluorene and 240 mL of anhydrous dimethyl sulfoxide. Under nitrogen protection, the temperature was raised to 60° C., and 69.11 g (0.50 mol) of anhydrous potassium carbonate was added. Maintain a constant temperature for nitro nucleophilic substitution reaction 24h. After cooling to room temperature, the reaction solution was poured into 2000 mL of 1 mol / L hydrochloric acid solution. Add 600mL chloroform for extraction. The organic phase was repeatedly washed with water until neutral, and dried by adding anhydrous magnesium sulfate. Chloroform was distilled off under reduced pressure to obtain a crude product. The crude product was washed...
Embodiment 2
[0066] Example 2, the synthesis of phenylethynyl-terminated PPQ (in formula I, Y=-) with a designed molecular weight of 5000 g / mol
[0067] 1) Tetraketone compound 9 shown in formula II, the synthesis of 9-bis[(4-benziloxy)phenyl]fluorene
[0068] Add 53.60g (0.21mol) of 4-nitrobenzil, 35.04g (0.10mol) of 9,9-bis[(4-hydroxyphenoxy ) phenyl] fluorene and 240 mL of anhydrous dimethyl sulfoxide. Under the protection of nitrogen, the temperature was raised to 60° C., and 62.19 g (0.45 mol) of anhydrous potassium carbonate was added. Maintain a constant temperature for nitro nucleophilic substitution reaction 24h. After cooling to room temperature, the reaction solution was poured into 2000 mL of 1 mol / L hydrochloric acid solution. Add 600mL chloroform for extraction. The organic phase was repeatedly washed with water until neutral, and dried by adding anhydrous magnesium sulfate. Chloroform was distilled off under reduced pressure to obtain a crude product. The crude product...
Embodiment 3
[0084] Example 3, the synthesis of phenylethynyl-terminated PPQ (in formula I, Y=-O-) with a designed molecular weight of 2500 g / mol
[0085] Add 13.1898g (17.20mmol) in a 250mL there-necked flask equipped with mechanical stirring, thermometer, nitrogen inlet, step 1) of embodiment 1) to prepare tetraketone compound 9 shown in gained formula II, 9-bis[(4-benzoic acid Acyloxy)phenyl]fluorene, 4.8415g (15.60mmol) 4-phenylethynyl benzil and 50g NMP, stirred at room temperature for 20min to mix well, then added 5.7568g (25.00mmol) 3,3',4, 4'-Tetraaminodiphenyl ether and 45 g NMP. . Under the protection of nitrogen, the temperature was raised to 120° C., and the reaction was carried out at constant temperature for 4 hours. After cooling to room temperature, the reaction solution was poured into water, and a yellow precipitate precipitated out. A yellow solid was obtained by filtration. The yellow solid was pulverized and then repeatedly washed with water and filtered. The obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com