Triphenylphosphine oxide connecting bisoxazoline ligand, preparation method and application thereof
A technology of triphenylphosphine and bisoxazoline, which is applied in the field of organic synthesis and chiral catalysts to achieve the effects of low reaction temperature, good catalytic activity and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
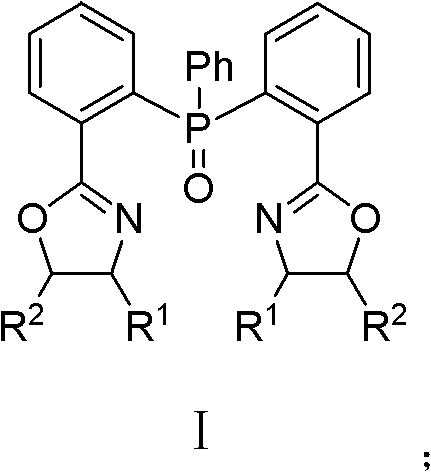
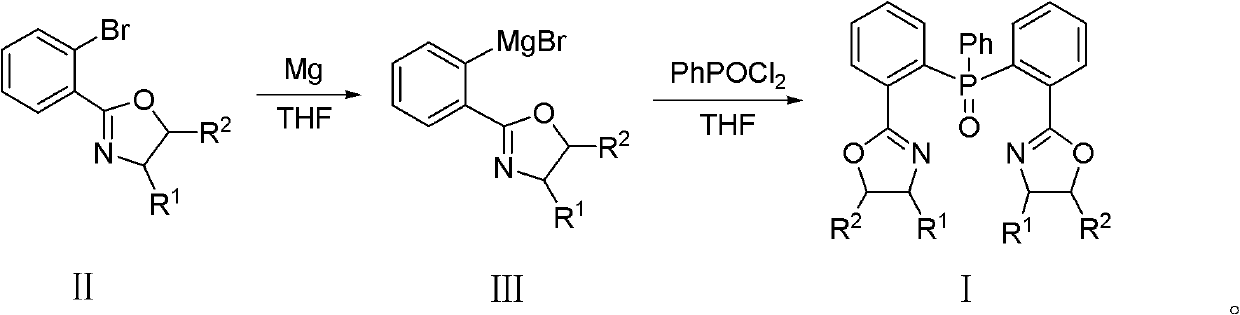
[0044] (4S, 4'S)-2,2'-[(phenylphosphorous) two-2,1-phenylene] bis[(4-benzyl)-2-oxazoline] preparation method, The method steps are as follows:
[0045] Under the protection of argon, in a 25ml three-necked flask equipped with a constant pressure dropping funnel and a condenser, add clean and dry magnesium chips (72mg, 3mmol), redistilled THF (2ml) and a small grain of iodine (20mg). At 50°C, a solution of (4S)-4-benzyl-2-(2-bromophenyl)oxazoline (948.6mg, 3mmol) in THF (2ml) was added dropwise; after the addition was complete, reflux for 2 hours, Get the corresponding Grignard reagent. After cooling to room temperature, a solution of phenylphosphoryl dichloride (195 mg, 1 mmol) in THF (2 ml) was added dropwise. After the addition was complete, the mixture was stirred at room temperature for 12 hours. The reaction was quenched with 10 ml of water, and then extracted with dichloromethane (3×10 ml) to separate the liquids. After the obtained organic phase 1 was washed with satu...
Embodiment 2
[0049] (4S, 4'S)-2,2'-[(phenylphosphorous) two-2,1-phenylene] bis[(4-isopropyl)-2-oxazoline] preparation method , the method steps are as follows:
[0050] Under nitrogen protection, in a 25ml three-neck flask equipped with a constant pressure dropping funnel and a condenser, add clean and dry magnesium chips (199mg, 8.29mmol), redistilled THF (2ml) and a small grain of iodine (10mg). At 50°C, a THF (2ml) solution of (4S)-4-isopropyl-2-(2-bromophenyl)oxazoline (2.222mg, 8.29mmol) was added dropwise; hours to obtain the corresponding Grignard reagent. After cooling to room temperature, a solution of phenylphosphoryl dichloride (538 mg, 2.76 mmol) in THF (2 ml) was added dropwise. After the addition was complete, the mixture was stirred at room temperature for 12 hours. The reaction was quenched with 10 ml of water, and then extracted with dichloromethane (3×10 ml) to separate the liquids. After the obtained organic phase 1 was washed with saturated sodium chloride solution an...
Embodiment 3
[0054] (4S, 4'S)-2,2'-[(phenylphosphorous) two-2,1-phenylene] bis[(4-tert-butyl)-2-oxazoline] preparation method , the method steps are as follows:
[0055] Under the protection of nitrogen, in a 25ml three-neck flask equipped with a constant pressure dropping funnel and a condenser, add clean and dry magnesium chips (68mg, 2.85mmol), redistilled THF (2ml) and iodine (30mg). At 50°C, a THF (2ml) solution of (4S)-4-tert-butyl-2-(2-bromophenyl)oxazoline (804.9mg, 2.85mmol) was added dropwise; hours to obtain the corresponding Grignard reagent. After cooling to room temperature, a solution of phenylphosphoryl dichloride (195 mg, 1 mmol) in THF (2 ml) was added dropwise. After the addition was complete, the mixture was stirred at room temperature for 12 hours. The reaction was quenched with 10 ml of water, and then extracted with dichloromethane (3×10 ml) to separate the liquids. After the obtained organic phase 1 was washed with saturated sodium chloride solution and separated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com