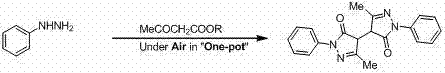Novel preparation method and use of bisedaravone and medicinal salts of bisedaravone
A technology of solvent and molar ratio, which is applied in the direction of drug combination, cardiovascular system diseases, antitoxic agents, etc., can solve the problems of long synthetic route, low yield and purity, and achieve the effect of preventing and treating cerebral infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of preparation method of diedaravone is in oxygen atmosphere, under reflux state, ethyl acetoacetate 130.1 g (1.00 mol) is added dropwise rapidly to phenylhydrazine 113.5 g (1.05 mol) and absolute ethanol 150 mL of the mixed solution, pay attention to stirring while adding dropwise, and the solid will precipitate out soon. After the addition, continue to stir and reflux for 6 h. Suction filtration while hot, wash with absolute ethanol (50 mL×3), and dry the filter cake to obtain a light yellow powdery solid, which is the crude product of diedaravone. Recrystallized with 1500 mL of absolute ethanol and decolorized with activated carbon to obtain 229.7 g of white powdery solid with a yield of 66.3%.
Embodiment 2
[0026] A kind of preparation method of diedaravone is in oxygen atmosphere, under reflux state, methyl acetoacetate 116.1 g (1.00 mol) is added dropwise rapidly to phenylhydrazine 113.5 g (1.05 mol) and absolute ethanol 150 mL of the mixed solution, pay attention to stirring while adding dropwise, and the solid will precipitate out soon. After the addition, continue to stir and reflux for 6 h. Suction filtration while hot, wash with absolute ethanol (50 mL×3), and dry the filter cake to obtain a light yellow powdery solid, which is the crude product of diedaravone. Recrystallized with 1500 mL of absolute ethanol and decolorized with activated carbon to obtain 215.1 g of white powdery solid with a yield of 62.1%.
Embodiment 3
[0028] A kind of preparation method of diedaravone, is in oxygen atmosphere, under reflux state, ethyl acetoacetate 130.1 g (1.00 mol) is added dropwise rapidly to phenylhydrazine 113.5 g (1.05 mol) and anhydrous methanol 150 mL of the mixed solution, pay attention to stirring while adding dropwise, and the solid will precipitate out soon. After the addition, continue to stir and reflux for 8 h. Suction filtration while hot, wash with anhydrous methanol (50 mL×3), and dry the filter cake to obtain a light yellow powdery solid, which is the crude product of diedaravone. Recrystallized with 1500 mL of absolute ethanol and decolorized with activated carbon to obtain 203.0 g of white powdery solid with a yield of 58.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com