Method for preparing high-optical purity pitavastatin calcium
A technology of pitavastatin calcium and optical purity, applied in the field of preparation of cholesterol-lowering drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
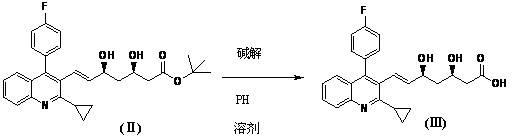
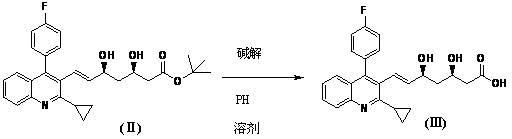
[0037] In a 1000ml four-neck flask, add 600ml methanol, 33g (0.0638mol) (3R,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline base]-3,5-dihydroxy-3,5-Oisopropylidene-6-heptenoic acid tert-butyl ester (I), at 10°C, add 85ml of 1mol / L hydrochloric acid, and react for 1 hour to obtain (3R ,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-6-(E)-heptenoic acid tert-butyl ester (Ⅱ) methanol solution .
[0038] Add 85ml of 1mol / L sodium hydroxide solution to the methanol solution of (II) above, stir for 2 minutes, then add 64ml of 1mol / L sodium hydroxide solution, and react for 2 hours. Recover under reduced pressure at 55°C, stop recovery when about 100-200ml remains, add water to 250ml, extract twice with 300ml dichloromethane each, adjust the acidity of the water layer to pH 4-5, extract with 300ml dichloromethane , washed with 50ml of purified water to obtain (3R,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-(E)-heptenoic acid (Ⅲ) i...
Embodiment 2
[0042] In a 1000 ml four-necked flask, add 600 ml of tetrahydrofuran, 33 g (0.0638 mol) (3R,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline base]-3,5-dihydroxy-3,5-Oisopropylidene-6-heptenoic acid tert-butyl ester (I), at 50°C, add 100ml of 1mol / L sulfuric acid, and react for 1 hour to obtain (3R ,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-(E)-heptenoic acid tert-butyl ester (Ⅱ) tetrahydrofuran solution .
[0043]Add 85 ml of 1 mol / L sodium hydroxide solution to the tetrahydrofuran solution of (II) above, stir for 2 minutes, then add 64 ml of 1 mol / L sodium hydroxide solution, and react for 2 hours. Recover under reduced pressure at 55°C, stop the recovery when there are about 100-200ml remaining, add water to 250ml, extract twice with 300ml methyl tert-butyl ether, adjust the acidity of the water layer to about 5, and use 300mM methyl Extracted with tert-butyl ether, washed with 50ml of purified water to obtain (3R,5S)-dihydroxy-7-[2-cyclo...
Embodiment 3
[0047] In a 1000ml four-neck flask, add 600ml of ethanol, 33g (0.0638mol) (3R,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline base]-3,5-dihydroxy-3,5-Oisopropylidene-6-heptenoic acid tert-butyl ester (Ⅰ), at 25°C, add 85ml of 1mol / L hydrochloric acid, and react for 1 hour to obtain (3R ,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-(E)-heptenoic acid tert-butyl ester (Ⅱ) ethanol solution .
[0048] Add 85ml of 1mol / L sodium hydroxide solution to the ethanol solution of (II) above, stir for 2 minutes, then add 64ml of 1mol / L sodium hydroxide solution, and react for 2 hours. Recover under reduced pressure at 55°C, stop recovery when about 100-200ml remains, add water to 250ml, extract twice with 300ml toluene each, adjust the acidity of the aqueous layer to pH 2-3, extract with 300ml dichloromethane, 50ml Purified and washed with water to obtain (3R,5S)-dihydroxy-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-6-(E)-heptenoic acid (Ⅲ ) in dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com