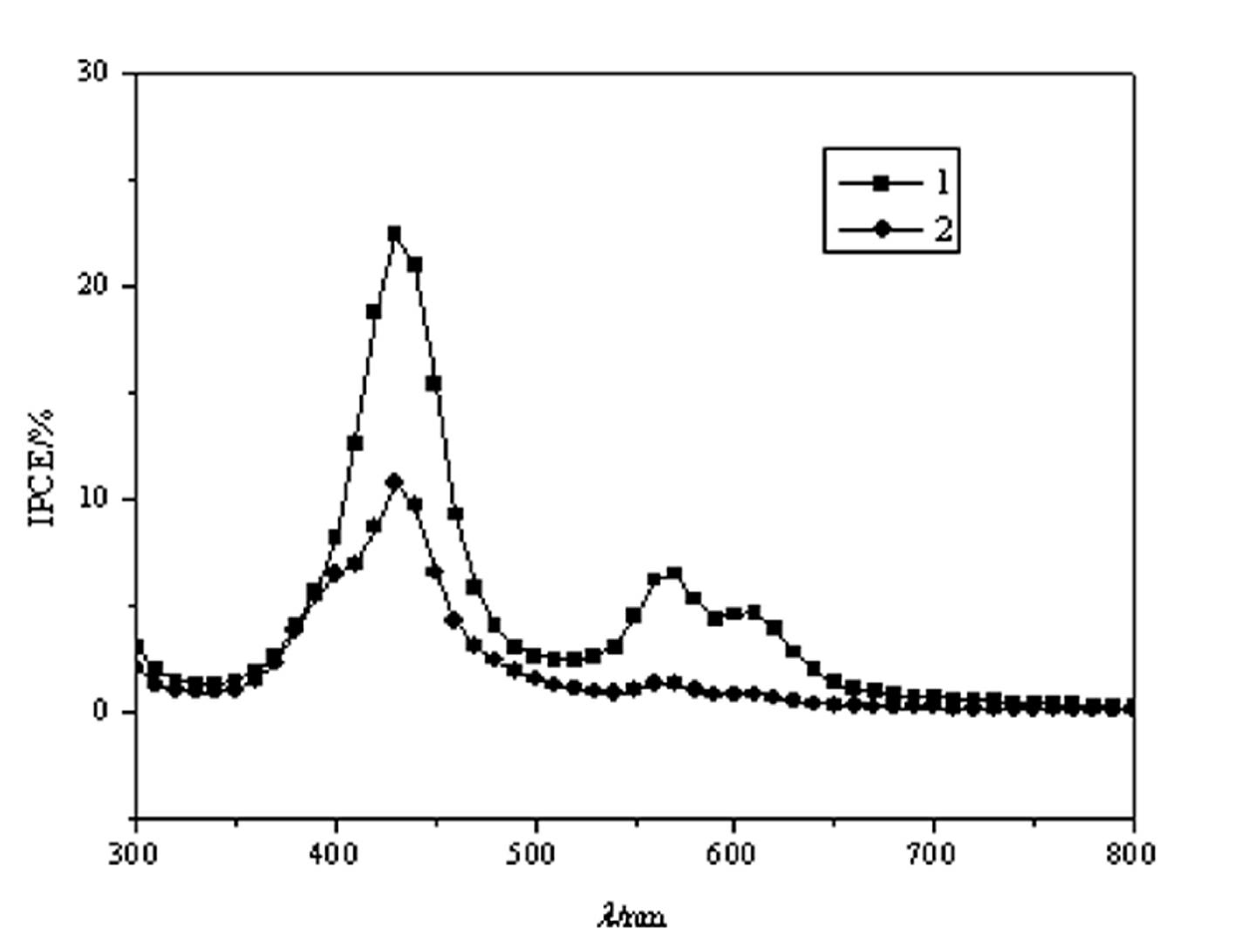
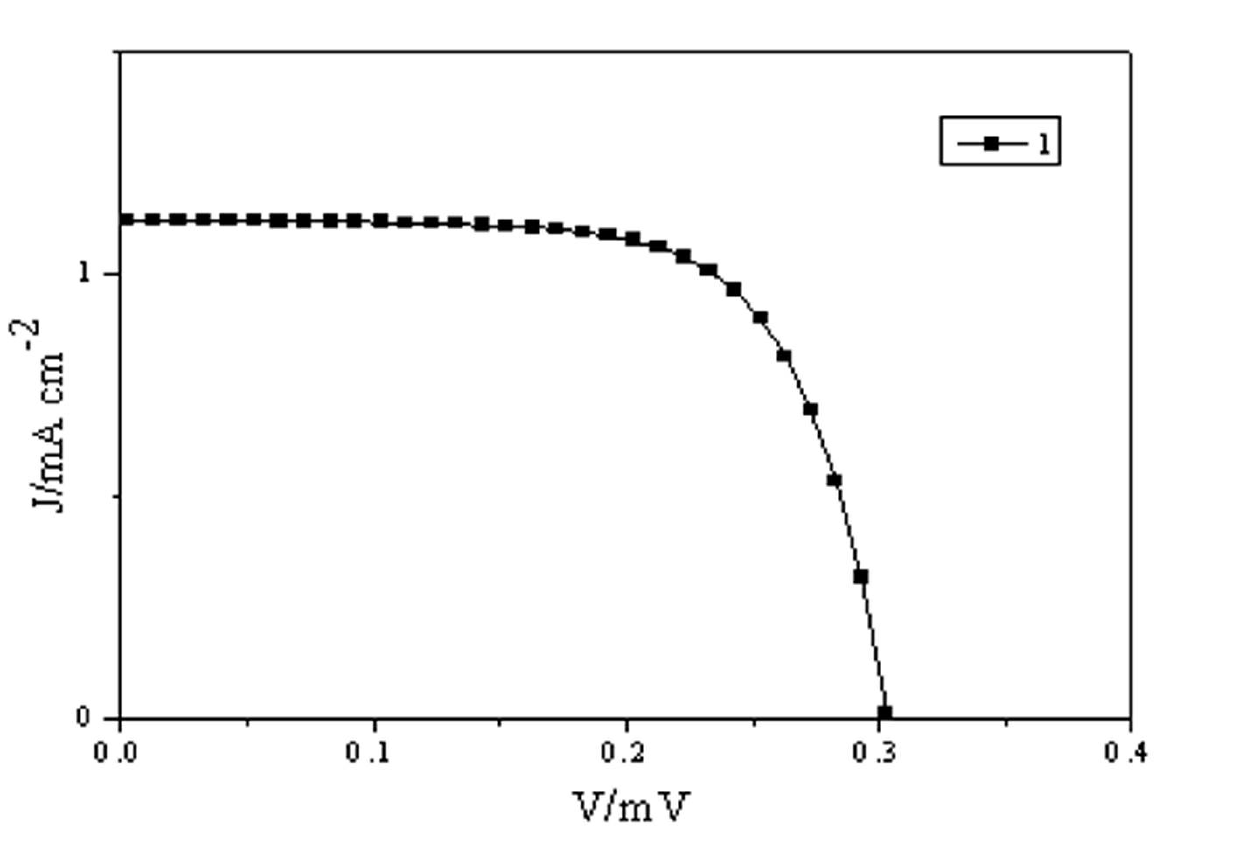
Zinc protoporphyrin containing heterocyclic ring as well as synthesis and applications of zinc protoporphyrin containing heterocyclic ring and metal complex of zinc protoporphyrin containing heterocyclic ring
A metal complex, zinc porphyrin technology, applied in the field of chemical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, photosensitizer 1 synthesis method
[0044] Add pyrrole and 4-bromobenzaldehyde to a mixed solvent of glacial acetic acid and nitrobenzene (the molar ratio of glacial acetic acid and nitrobenzene is 1:2) at a molar ratio of 1:1, heat to 140°C and reflux for 1 hour, After completion of the reaction, cool to room temperature and stir for 12 hours, filter, separate by column chromatography, use silica gel as the stationary phase, and collect the main color bands to obtain 5,10,15,20-tetrakis(4-bromophenyl)porphyrin.
[0045] 5,10,15,20-tetrakis(4-bromophenyl)porphyrin and zinc acetate were reacted at 65°C for 5 hours at a molar ratio of 1:5 to obtain 5,10,15,20-tetrakis(4- bromophenyl) zinc porphyrin.
[0046] Add 5,10,15,20-tetrakis(4-bromophenyl)zinc porphyrin and imidazole to anhydrous DMF solution at a molar ratio of 1:8, then add 5,10,15,20-tetrakis(4 -Bromophenyl) copper powder with 5 times the molar weight of zinc porphyrin and potassium carbonate w...
Embodiment 2
[0049] Embodiment 2, photosensitizer 2 synthesis method
[0050] Add pyrrole and 4-bromobenzaldehyde to a mixed solvent of glacial acetic acid and nitrobenzene in a molar ratio of 1:1 (the molar ratio of glacial acetic acid and nitrobenzene is 1:2), reflux at 140°C for 1 hour, and react After completion, cool to room temperature and stir for 12 hours, filter, separate by column chromatography, use silica gel as the stationary phase, and collect the main color bands to obtain 5,10,15,20-tetrakis(4-bromophenyl)porphyrin.
[0051] 5,10,15,20-tetrakis(4-bromophenyl)porphyrin and zinc acetate were reacted at 65°C for 5 hours at a molar ratio of 1:5 to obtain 5,10,15,20-tetrakis(4- bromophenyl) zinc porphyrin.
[0052] Add 5,10,15,20-tetrakis(4-bromophenyl)zinc porphyrin and benzimidazole at a molar ratio of 1:8 to anhydrous DMF solution, then add 5,10,15,20-tetra (4-Bromophenyl) zinc porphyrin molar amount of 5 times the copper powder and bromophenyl zinc porphyrin molar amount ...
Embodiment 3
[0055] Embodiment 3, the synthesis of the mercury complex of photosensitizer 1
[0056] Dissolve photosensitizer 1 (0.05mmol) in 4ml of chloroform to form a solution of photosensitizer 1; drop mercuric chloride (0.1mmol) in methanol (4ml) into the solution of photosensitizer 1 to precipitate a precipitate, namely It is the mercury complex of photosensitizer 1.
[0057] Characterization of Photosensitizer Photosensitizer 1 Mercury Complex:
[0058] UV-Vis (CHCl 3 ) λnm : 429 (Soret band), 561, 600 (Q band).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com