Fluorescent probe for detecting hypochlorous acid and preparation method thereof
A technology for detecting hypochlorous acid and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of not being able to truly apply to biological systems, poor water solubility, etc., and achieve rapid action, excellent performance, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation method of the fluorescent probe that detects hypochlorous acid
[0032] In a 25mL round bottom flask, add dimethylaminothioformyl chloride (1.26g, 10.0mmol), dissolve it in 6.0mL of dry DMF, add 7-hydroxyphenoxazinone sodium salt (0.6g, 2.5mmol ) and potassium carbonate (0.52g, 3.8mmol), stirred at room temperature for 30h under nitrogen atmosphere. The obtained brownish-yellow slurry was diluted with 350-400mL saturated sodium chloride solution, and a red precipitate was precipitated by salting out. After vacuum drying, the silica gel column separation (CH 2 Cl 2 as eluting agent) to obtain 0.375 g of red powder (target product, i.e. a fluorescent probe for detecting hypochlorous acid). (Yield 50%).
[0033] ESI-MS: C 15 h 12 N 2 o 3 S, m / z: 301.1 (M+1) + .
[0034] 1 H NMR (CDCl 3 ): δ3.388 (s, 3H, NCH 3 ), 3.477 (s, 3H, NCH 3 ), 6.331(d, J=1.2Hz, 1H, ArH), 6.866(q, J=6.0Hz, 1H, ArH), 7.093(q, J=3.4Hz, 1H, ArH), 7.120(d, J= ...
Embodiment 2
[0036] Example 2: Description of the chemical properties of the fluorescent probe for detecting hypochlorous acid and its application in microscopic fluorescence imaging of endogenous HClO in cells
[0037] 1. Probe in neutral aqueous solution 1 The detection performance test of:
[0038] (1) Preparation steps of the test solution:
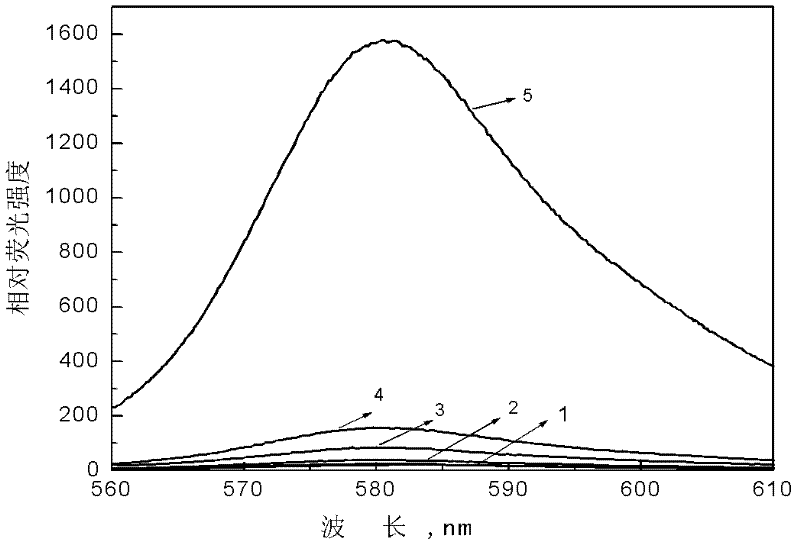
[0039] In a 10mL graduated test tube, sequentially add 5.0mL of pH 7.40 phosphate buffer solution with a concentration of 0.2mol / L, a certain amount of standard NaClO solution (accurately calibrated by spectrophotometry in advance, see literature: M.Mazda and D.W.Margerum.Inorg .Chem.1994, 33, 118.), diluted to the mark with three times distilled water. Shake well. Add 0.1 mL to a concentration of 1.0 x 10 -3 mol / L methanol stock solution of the fluorescent probe for detecting hypochlorous acid, shake well. Make relevant measurements immediately. The fluorescent probe solution for detecting hypochlorous acid, which is operated as above, but ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com