Immobilization method of proteinase molecule by using nano-material and application thereof
A technology of nanomaterials and proteases, applied in the direction of immobilized on or in inorganic carriers, nanotechnology, nanomedicine, etc., can solve the problems of low catalytic efficiency and achieve the effect of simple production process, low price and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Activation of multi-walled carbon nanotubes (MWNTs) and immobilization of SOD enzyme.
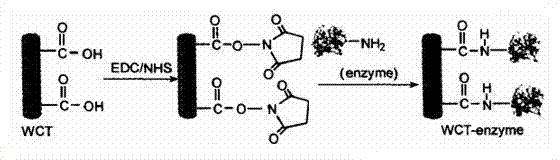
[0023]Pour 0.5g of commercial multi-walled carbon nanotubes into the mixed acid of 15ml of concentrated nitric acid and 45mL of concentrated sulfuric acid, seal the container, vibrate with ultrasonic wave for 3h respectively; Multi-walled carbon nanotubes were washed repeatedly with deionized water until neutral, dried in vacuum at 100°C and weighed. Take 50 mg of dried acid-treated MWNTs, add them into 15 ml of phosphate buffer solution with pH=7.0, and disperse them evenly by ultrasonic; then add 200 mg of carbodiimide (EDC) and N-hydroxysuccinimide (NHS ) 250 mg, sonicate for 30 min; add a certain amount of enzyme solution to the above solution and shake at room temperature for 24 h; then filter with a 0.22 μm polycarbonate membrane; the obtained solid is washed with phosphate buffer, and then dried in vacuum to obtain the final fixation Catase.
[0024] First use mix...
Embodiment 2
[0029] Example 2: Functionalized multi-walled carbon nanotubes are used for lipase immobilization.
[0030] Carboxyl groups are attached to the surface of the multi-walled carbon nanotubes after the mixed acid treatment of concentrated nitric acid and concentrated sulfuric acid at a certain ratio (volume ratio 1:2~1:5), and then thionyl chloride and N,N-dimethylformamide Acyl chlorination is carried out to obtain chlorine-containing acyl multi-walled carbon nanotubes, and then the chlorine-containing acyl multi-walled carbon nanotubes are reacted with hexamethylenediamine in chloroform to obtain polyamino-terminated multi-walled carbon nanotubes, namely CNT-NH. Acidified carbon nanotubes react with hexadecyl bromide to obtain CNT-NH under certain conditions.
[0031] Functionalized carbon nanotubes were sonicated in Tris-HCl buffer solution, then added lipase solution prepared by genetic engineering and centrifuged by shaking. The amount of immobilized enzyme was determined b...
Embodiment 3
[0033] Example 3: Immobilization of SOD enzyme by single-walled carbon nanotubes (SWNTs).
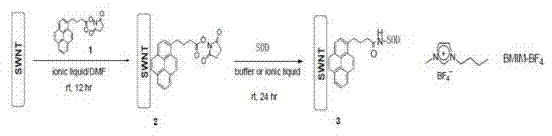
[0034] Suspend 6mg of SWNTs in 6mlBMIM-BF4 (an ionic liquid) to obtain liquid A; then add 60mg of N-hydroxysuccinimide ester 1-pyrenebutyric acid to 12ml of DMF (dimethylformamide) to obtain liquid B; add liquid A to Liquid B was mixed and shaken at room temperature for 12 hours; then 30ml of methanol was added, and the functionalized SWNTs would precipitate; the precipitate was washed three times with pure methanol, 50ml each time, to remove excess reducing agent; dried under vacuum to obtain functionalized SWNTs; 5mg of functionalized SMNTs was suspended in 5ml of BMIM-BF4 and then added to SOD solution (54mg of enzyme was dissolved in 10ml of 20mM pH 7.2 phosphate buffer); the above heterogeneous mixture was shaken at room temperature for 24h; Filtration; the finally obtained solid was washed with phosphate buffer (25mlx2), then dried in vacuo to obtain the final immobilized enzyme. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com