Urea sulfate and preparation method thereof
A technology of urea sulfate and sulfuric acid solution, applied in the preparation of urea derivatives, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of health impact, complex production process, impact on production output, etc., and achieve outstanding energy saving and cost saving. , The effect of a wide range of raw materials and mild processing conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
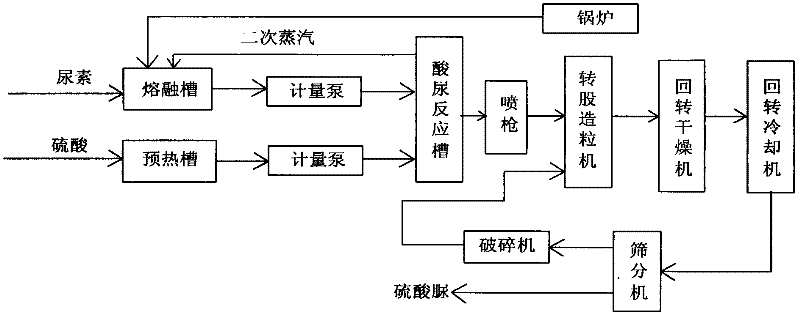
[0030] Such as figure 1 As shown, the preparation steps of urea sulfate of the present invention are as follows:
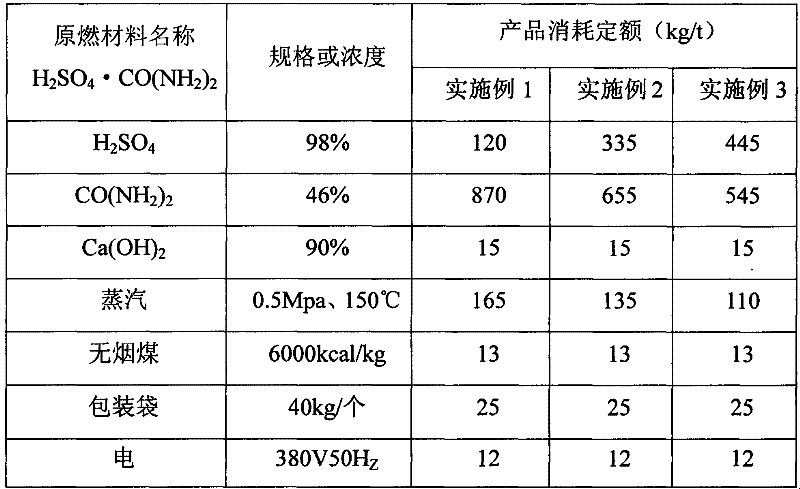
[0031] (1) A urea solution with a concentration of 46% is prepared through a melting tank at a melting temperature of 117.7°C. In order to ensure the preparation effect, during the preparation process of the urea solution, steam with a temperature of 150° C. and a pressure of 0.5 Mpa needs to be introduced for heating. The steam is preferably prepared in a boiler using anthracite as fuel.
[0032] At the same time, a sulfuric acid solution with a concentration of 98% is prepared through a preheating tank at a preheating temperature of 95°C.
[0033] Among them, the preheating temperature of the sulfuric acid solution is set to 95°C in order to achieve the crystallization effect when mixed with the heated urea solution, thereby obtaining a better granulation effect. The setting of water vapor is to promote the combination of urea solution and sulfuric acid solution, ma...
Embodiment 2
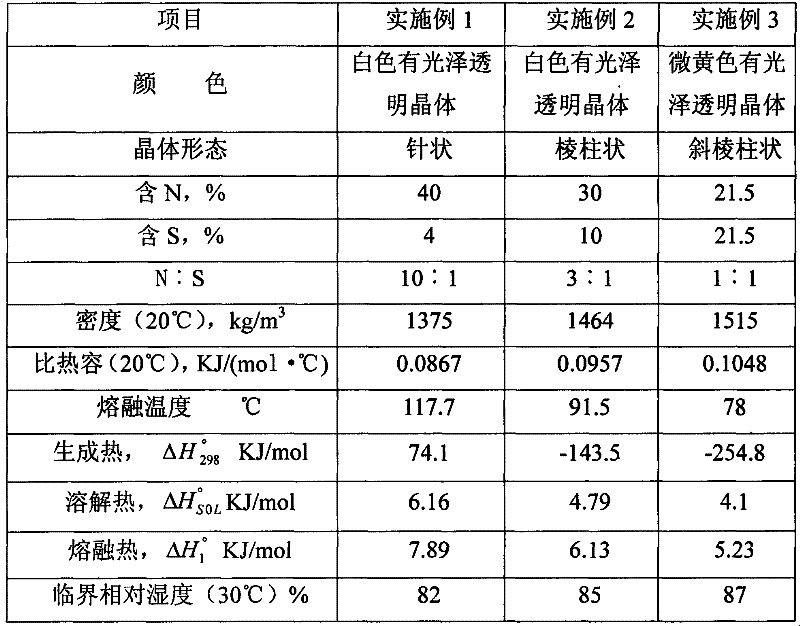
[0042] Compared with Example 1, the preparation process of urea sulfate in this example is the same. The difference lies in the temperature during the reaction and the mass ratio of the urea solution to the sulfuric acid solution. The mass ratio of the urea solution to the sulfuric acid solution used in this example is 3.19:1, the urea melting temperature is 91.5°C, and the preheating temperature of sulfuric acid is 75°C, so the prepared urea sulfate product is a white glossy transparent prism The mass ratio of nitrogen and sulfur is 3:1.
[0043] At this time, the pH value of the 0.1mol / L solution of the entire urea sulfate product is 7.2, the mass fraction of nitrogen ≥ 30%, the mass fraction of sulfur ≥ 10%, the mass fraction of free acid ≤ 0.05%, and the mass fraction of biuret The fraction is less than or equal to 1.5%, the mass fraction of water is less than or equal to 1%, the mass fraction of a single element of a middle element is greater than or equal to 2%, and the mas...
Embodiment 3
[0045] Compared with Example 1, the preparation process of urea sulfate in this example is the same. The difference lies in the temperature during the reaction and the mass ratio of the urea solution to the sulfuric acid solution. The mass ratio of the urea solution to the sulfuric acid solution used in this example is 2:1, the urea melting temperature is 78°C, and the preheating temperature of sulfuric acid is 65°C, so the prepared urea sulfate product is slightly yellow, shiny and transparent. Oblique prismatic crystals have a mass ratio of nitrogen and sulfur of 1:1.
[0046] At this time, the pH value of the 0.1 mol / L solution of the entire urea sulfate product is 6.5, the mass fraction of nitrogen is ≥ 21.5%, and the mass fraction of sulfur is ≥ 21.5%. , The mass fraction of free acid ≤ 0.2%, the mass fraction of biuret ≤ 1.5%, the mass fraction of water ≤ 1%, the mass fraction of a single element of middle element ≥ 2%, the mass fraction of a single component of trace eleme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Resistance to crushing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com