Ganciclovir eye drops and preparation method thereof
A technology of ganciclovir and eye drops, which is applied in the field of medicine, can solve problems such as poor water solubility, easy crystallization, and affecting use, and achieve the effects of less systemic side effects, strong buffering capacity, and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Excipient compatibility investigation
[0017] 1: Ganciclovir 0.1g, water for injection 100ml
[0018] 2: Ganciclovir 0.1g, Tween-800.1g, water for injection 100ml
[0019] 3: Ganciclovir 0.1g, poloxamer 10g, water for injection 100ml
[0020] 4: Ganciclovir 0.1g, Mannitol 5g, Water for injection 100ml
[0021] 5: Ganciclovir 0.1g, sodium chloride 0.9g, water for injection 100ml
[0022] 6: Ganciclovir 0.1g, boric acid buffer system, water for injection 100ml
[0023] 7: Ganciclovir 0.1g, phosphate buffer system, water for injection 100ml
[0024] 8: Ganciclovir 0.1g, 5% benzalkonium bromide 1.5g, water for injection 100ml
[0025] 9: Ganciclovir 0.1g, 5% benzalkonium bromide 0.2%, water for injection 100ml
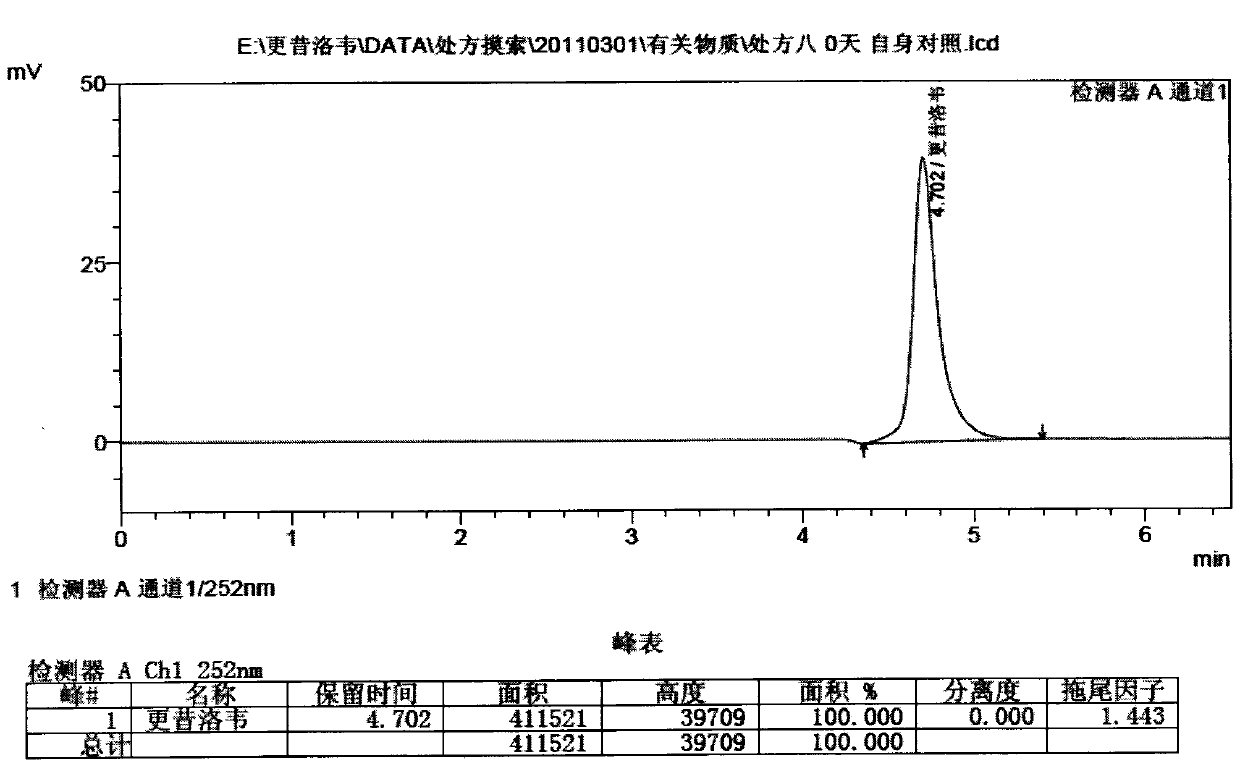
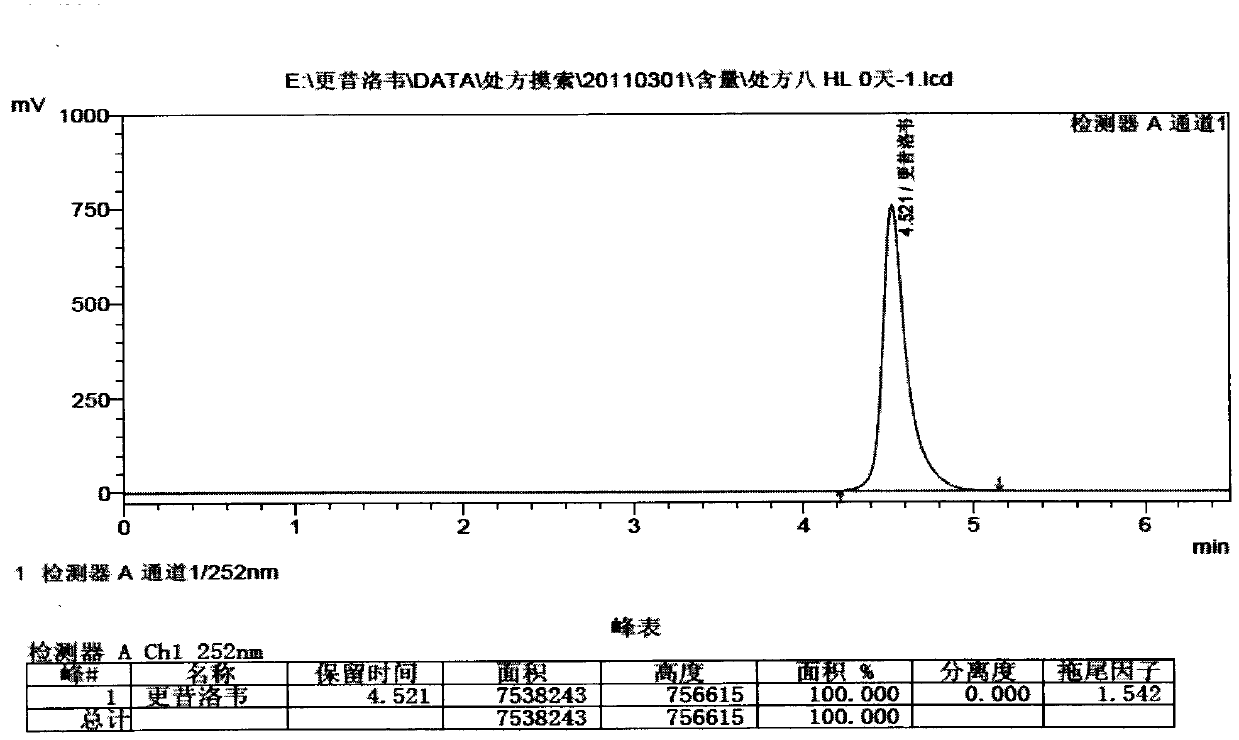
[0026] Measure the pH value after the above prescription is equipped, the list is as follows
[0027]
[0028] Table 1
[0029] It can be seen from the table that the other auxiliary materials except borate buffer system and phosphate buff...
Embodiment 2
[0031]
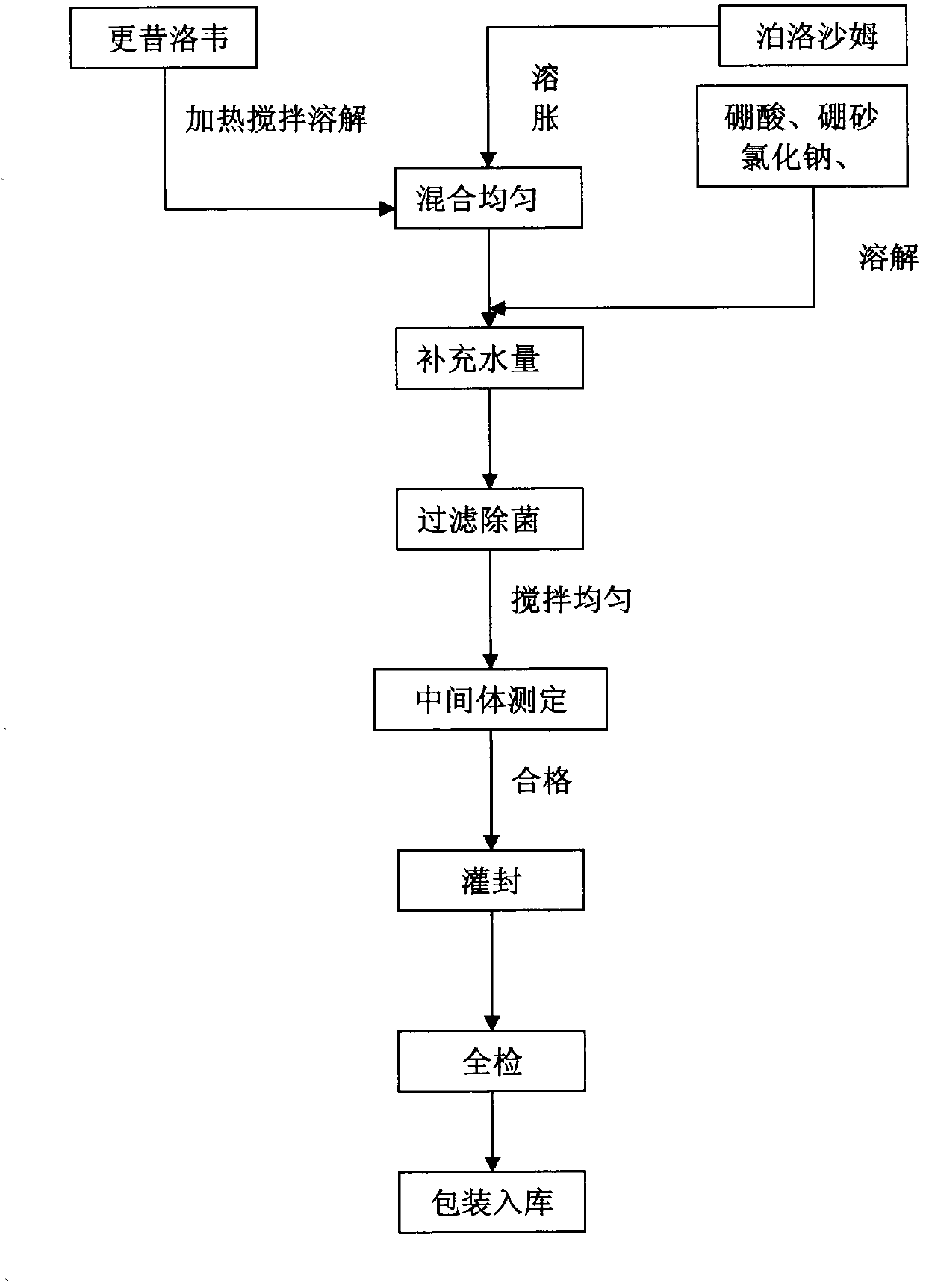
[0032] Steps:
[0033] 1. Weigh ganciclovir, add water 150ml, add Tween-800.2g, stir to dissolve;
[0034] 2. Weigh 10g of mannitol and add it to the above solution, stir to dissolve it;
[0035] 3. Weigh boric acid and borax to the above solution, stir and dissolve, then add benzalkonium bromide;
[0036] 4. Add water for injection, dilute to 200ml, shake well;
[0037] 5. Microporous membrane filtration sterilization, packaging.
Embodiment 3
[0039]
[0040] Steps:
[0041] 1. Weigh 10g of poloxamer, add 100ml of cold water to dissolve;
[0042] 2. Weigh 0.2g of ganciclovir, add 50ml of water for injection to dissolve;
[0043] 3. Mix the above two solutions and add phosphate buffer;
[0044] 4. Add mannitol and benzalkonium bromide to the above solution, stir to dissolve and mix;
[0045] 5. Add water for injection, dilute to 200ml, shake well;
[0046] 6. Microporous membrane filter sterilization, packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com