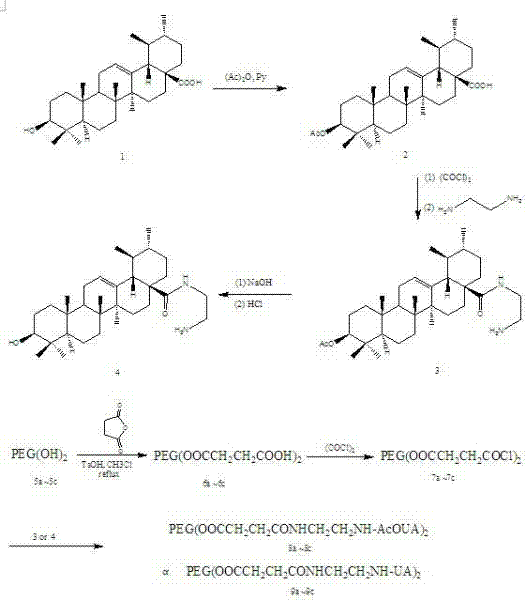
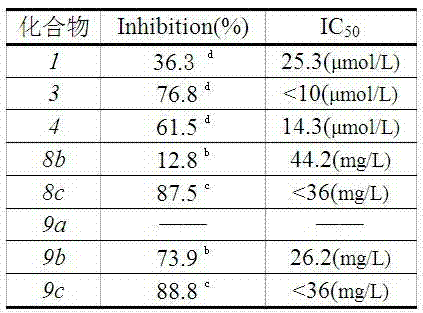
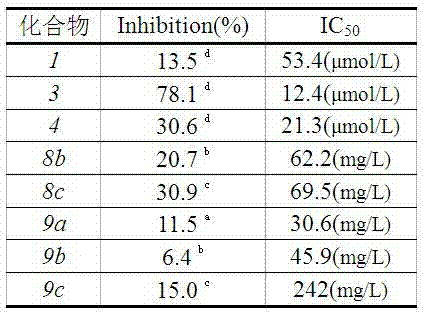
Ursolic acid derivative chemically modified by polyethylene glycol and preparation method of ursolic acid derivative
A polyethylene glycol, chemical modification technology, applied in organic chemistry, steroids, medical preparations containing active ingredients, etc., can solve the problem of reducing the anti-tumor activity of derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] compound 2 Preparation of:
[0042] 10 g of ursolic acid was dissolved in 45 mL of pyridine, and 25 mL of acetic anhydride was slowly added dropwise in an ice bath, and the reaction was carried out at room temperature for 8 h after dropping. Stop the reaction, evaporate pyridine under reduced pressure, dissolve the residue with dichloromethane, wash the organic phase with hydrochloric acid (5%), distilled water, and saturated sodium chloride solution successively, and filter the organic phase with anhydrous sodium sulfate to remove water for 2 h. The filtrate was concentrated under reduced pressure at 45°C and then dried to constant weight. The obtained crude product was 10.50 g. The crude product was recrystallized from absolute ethanol to obtain 8.59 g of white needle-like crystals. The crystallization yield was 83.3%, and the total yield was 80.5%. mp: 282~283°C.
[0043] 1 H NMR (600MHz, CDCl 3 ): 5.32(t, 1H, J =3.6 Hz, H-12), 4.42 (t, 1H, J =5.6 Hz, H-3), 2...
Embodiment 2
[0098] a: Dissolve 8 g of ursolic acid in 50 mL of pyridine, slowly add 20 mL of acetic anhydride dropwise in ice bath, and react at room temperature for 9 h after dropping; stop the reaction, evaporate pyridine under reduced pressure, and dissolve the residue in dichloromethane , and successively wash the organic phase with 5% hydrochloric acid, distilled water, and saturated sodium chloride solution, and then filter the organic phase with anhydrous sodium sulfate for 1 h. The filtrate is concentrated under reduced pressure at 50°C and dried to constant Weight, the crude product was obtained, and the crude product was recrystallized with absolute ethanol to obtain compound 2;
[0099] b: 4 g of compound 2 was completely dissolved in 50 mL of dichloromethane, and 4 mL of oxalyl chloride was slowly added dropwise in an ice bath. After the dropwise addition, continued to stir in an ice bath for 0.5 h, and then continued to react at room temperature for 12 h; After the reaction w...
Embodiment 3
[0107] a: Dissolve 12 g of ursolic acid in 40 mL of pyridine, slowly add 30 mL of acetic anhydride dropwise in an ice bath, and react at room temperature for 6 h after dropping; stop the reaction, evaporate pyridine under reduced pressure, and dissolve the residue in dichloromethane , and successively washed the organic phase with 5% hydrochloric acid, distilled water, and saturated sodium chloride solution, and then filtered the organic phase with anhydrous sodium sulfate for 3 h. The filtrate was concentrated under reduced pressure at 40°C and dried to constant Weight, the crude product was obtained, and the crude product was recrystallized with absolute ethanol to obtain compound 2;
[0108] b: 6 g of compound 2 was completely dissolved in 30 mL of dichloromethane, and 7 mL of oxalyl chloride was slowly added dropwise under ice bath conditions. After the dropwise addition, continued to stir in the ice bath for 2 h, and then continued to react at room temperature for 36 h; A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com