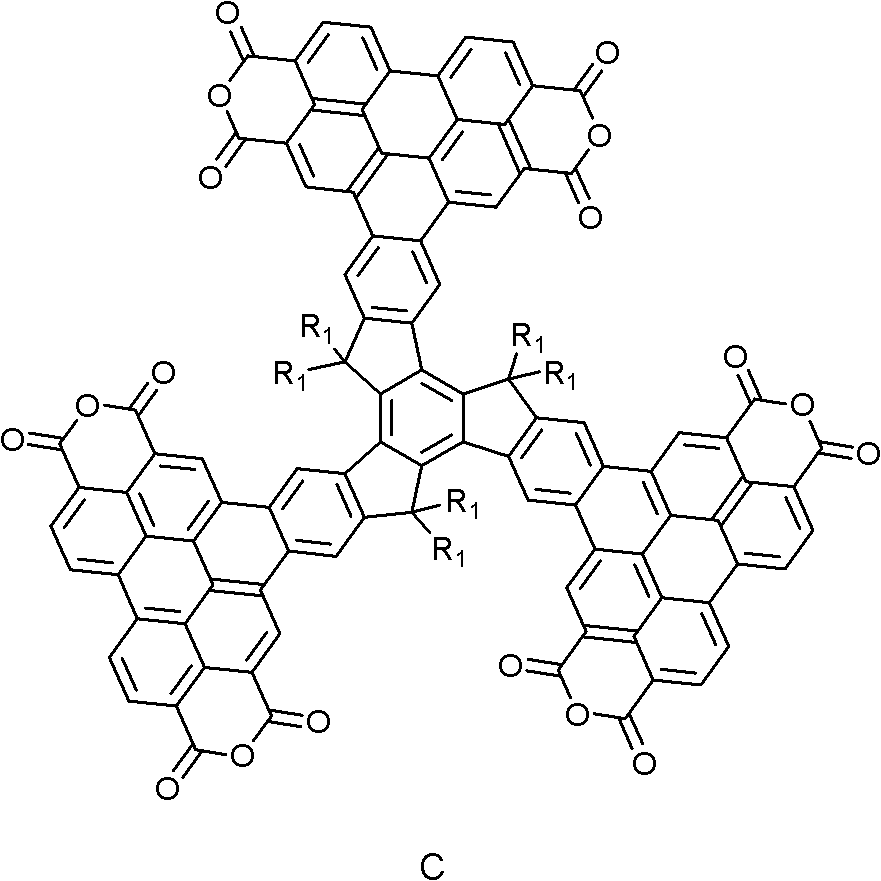
Star molecule of truxene-perylene-series derivative and preparation method thereof
A technology of trisindene and its derivatives, which is applied in the fields of organic and fine chemicals, can solve problems such as the scarcity of star-shaped N-type organic semiconductors, and achieve the effects of improving solubility, thermal stability, and film formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 intermediate 1
[0029]
[0030] In a 50mL flask, add 400mg of tripolyindene borate (compound Tr), 610mg of N,N'-bis(3-pentyl)-1-bromo-3,4,9,10 perylene imide, and Potassium 800mg, Pd(PPh 3 ) 2 Cl 2 40mg, chloroform 25mL, under argon atmosphere, heated to reflux for 6h. After the reaction was completed, 475 mg of a red solid was obtained through column chromatography separation, with a yield of 60%. 1 H-NMR (400MCDCl 3 ): δ=8.69(m, 18H), 8.19-8.06(m, 6H), 7.66(s, 3H), 7.54(s, 3H), 5.30(m, 6H), 3.03(m, 6H), 2.30- 1.57(m, 30H), 1.06-0.57(m, 96H).
Embodiment 2
[0031] The preparation of embodiment 2 compound 2
[0032]
[0033] In a 250mL flask, add compound 1 475mg, 100mL tetrahydrofuran, solar simulator (AM1.5, 100mW / cm 2 ) irradiation, add elemental iodine 5mg, react at room temperature for 6h. Tetrahydrofuran was distilled off under reduced pressure, and 426 mg of compound 2 was obtained by column chromatography, with a yield of 90%. 1 H-NMR (400M CDCl 3 ): δ=10.62(d, 9H), 9.67(s, 3H), 9.35(d, 6H), 9.12(d, 6H), 5.38(m, 6H), 3.82(m, 6H), 3.15(m, 6H), 2.54(m, 12H), 2.18(m, 12H), 1.18-0.72(m, 84H), 0.37(m, 18H).
Embodiment 3
[0034] The preparation of embodiment 3 compound 3
[0035]
[0036] In a 50mL flask, add tripolyindene borate (compound Tr) 400mg, 1-bromoperylenetetracarboxylate 832mg, potassium hydroxide 820mg, Pd (PPh 3 ) 2 Cl 2 40mg, chloroform 25mL, under argon atmosphere, heated to reflux for 6h. After the reaction was completed, 3540 mg of the compound was obtained through column chromatography separation, with a yield of 60%. 1 H-NMR (400M CDCl 3 ): δ=8.46(s, 3H), 8.35(m, 6H), 8.21(s, 3H), 8.15(m, 6H), 7.96(s, 3H), 7.58(m, 3H), 7.48(m, 6H ), 4.38-4.23(m, 24H), 2.98(s, 6H), 2.01-1.79(m, 30H), 1.69-1.39(m, 24H), 1.26-0.93(m, 72H), 0.66(m, 30H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com