Uncharged amphiphilic chitosan nano drug carrier and preparation method and application thereof
A technology of chitosan nano and chitosan derivatives, which is applied in drug combination, antineoplastic drugs, powder delivery, etc. It can solve the problems of unfavorable controlled release, poor stability, difficult application, etc., and achieve slow drug release and easy operation , the effect of controllable output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) At room temperature, 4g chitosan (24KDa) was dissolved in 200mL hydrochloric acid aqueous solution, then the pH of the solution was adjusted to 5.6 with 1N NaOH to form a transparent 2% (w / v) chitosan solution.
[0033] 2) Get a certain amount of deoxybile acid, make the ratio of the amount of substance of deoxybile acid to the amount of substance of glucosamine residue in chitosan be 0.1: 1, join it in the DMSO solution of 100mL, then add deoxybile acid EDC and NHS were added into the solution in equal amounts, and the ratio of EDC to deoxybile acid was kept constant at 1.2:1. Deoxybile acid, EDC and NHS were reacted at room temperature at 1000rpm for 30min.
[0034] 3) A mixed solution of lithocholic acid, EDC and NHS reacted in 100 mL of DMSO for 30 min was slowly added dropwise to a 2% (w / v) chitosan solution within 30 min, and reacted for 24 h at a stirring speed of 1000 rpm.
[0035] 4) The above 300mL reaction mixture solution was added into 200mL methanol, a...
Embodiment 2
[0044] Except that the mol ratio of deoxyacid and glucosamine residue in chitosan is 0.2: 1, other conditions are the same as specific example 1.
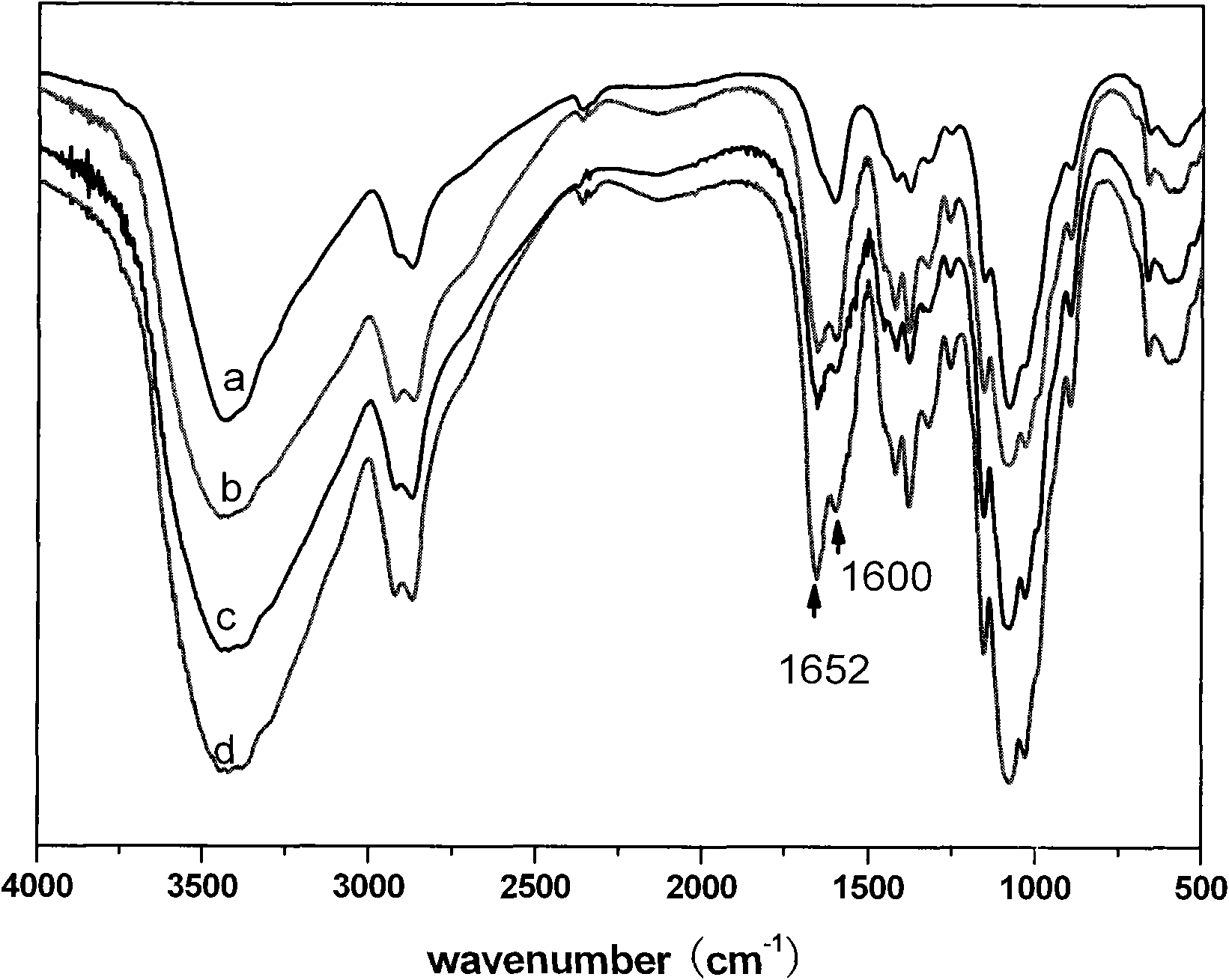
[0045] The FTIR characterization spectrum of the synthetic product of the first step of grafting deoxybile acid under this condition is shown in figure 1 -c.
[0046] The FTIR characterization spectrum of the synthetic product of the second step grafted glyceryl trimethylammonium chloride under this condition is shown in figure 2 -c.
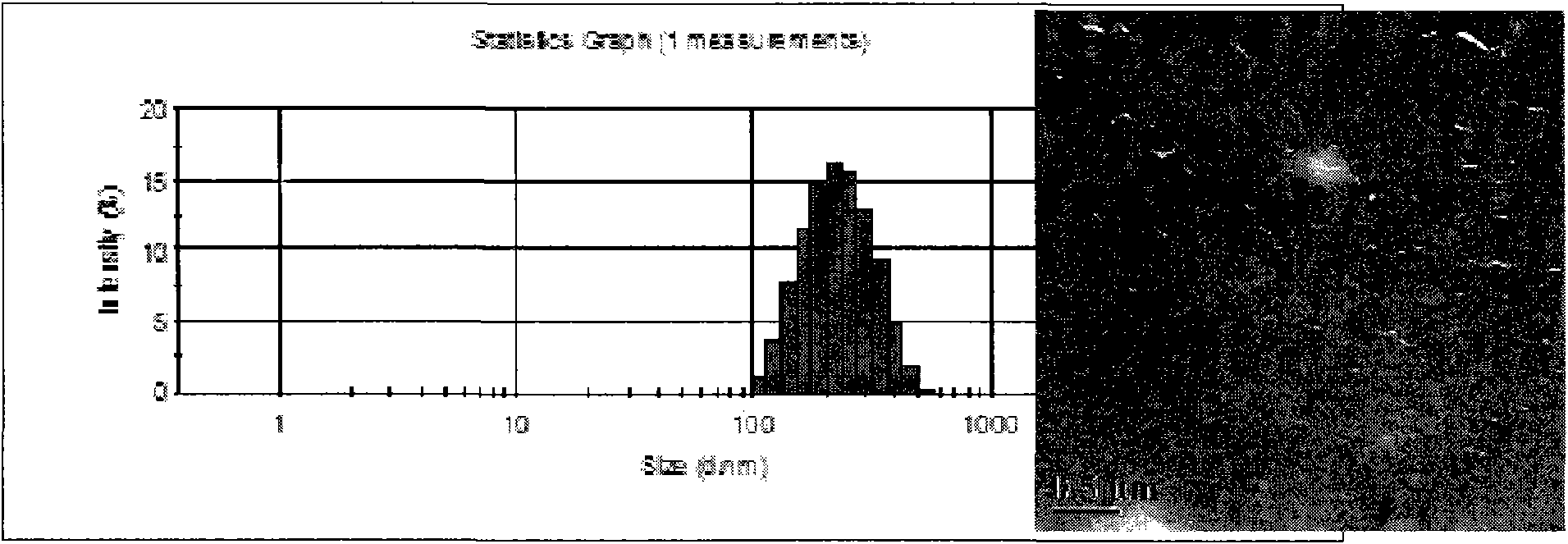
[0047] The particle size intensity distribution diagram and TEM morphology characterization diagram of the nanoparticles prepared under this condition are shown in image 3 (Z-average=201.0±3.0 nm, PDI=0.151).
[0048] The drug-loading capacity and drug-loading efficiency of the materials prepared under this condition were 17.3% and 88%, respectively.
[0049] The release curve of the drug-loaded nanoparticles prepared under the condition of pH=7.4 is shown in Figure 4 b.
Embodiment 3
[0051] Except that the molar ratio of deoxybile acid to glucosamine residue in chitosan is 0.3:1, other conditions are the same as in specific example 1.
[0052] The FTIR characterization spectrum of the synthetic product of the first step of grafting deoxybile acid under this condition is shown in figure 1 -d.
[0053] The drug-loading capacity and drug-loading efficiency of materials prepared under this condition to load PTX were 18.3% and 91.5%, respectively.
[0054] The release curves of the drug-loaded nanoparticles prepared under the conditions of pH=6.0 and pH=7.4 are shown in Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| critical micelle concentration (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com