A kind of α-type conus polypeptide and its application
A sequence and amino acid technology, applied in the field of α-type conus polypeptide, can solve the problems of poor therapeutic effect and tolerance of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1. Discovery of α-type Cono polypeptide
[0082] 1. Extraction of Total RNA from Cono
[0083] Six species of Cono snails were collected from the Paracel Islands, Sanya, Hainan and other places as sample materials, and the total RNA of the six species of Cono snails was extracted by the Trizol method. 6 types of Conus: Conus marmoreus (Conus marmoreus), Conus imperialis (Conus imperialis), Conus litteratus (Conus litteratus), Conus emaciatus (Conus emaciatus), Conus eberneus, Conus (conus) Conus vitulinus).
[0084] The specific steps of the Trizol method to extract total RNA:
[0085] ① Take the cono venom gland tube on ice, freeze it in liquid nitrogen and grind it into powder with a grinder, and pour it into a micro-homogenizer in an ice bath.
[0086] ②Pick 1.2ml Trizol into a homogenizer and further grind it to lyse the cono tissue to release RNA.
[0087] ③Pour the ground slurry into a 1.5ml centrifuge tube and let it stand at room temperature for 5 minutes.
[0088...
Embodiment 2
[0115] Example 2. Synthesis of α-type Cono polypeptide
[0116] The seven polypeptides shown in Table 3 were synthesized separately.
[0117] Table 3 Amino acid sequence of α type Cono polypeptide
[0118] Name
Sequence
T-1
NH 2 -GCCSNPACMLKNPNLC*
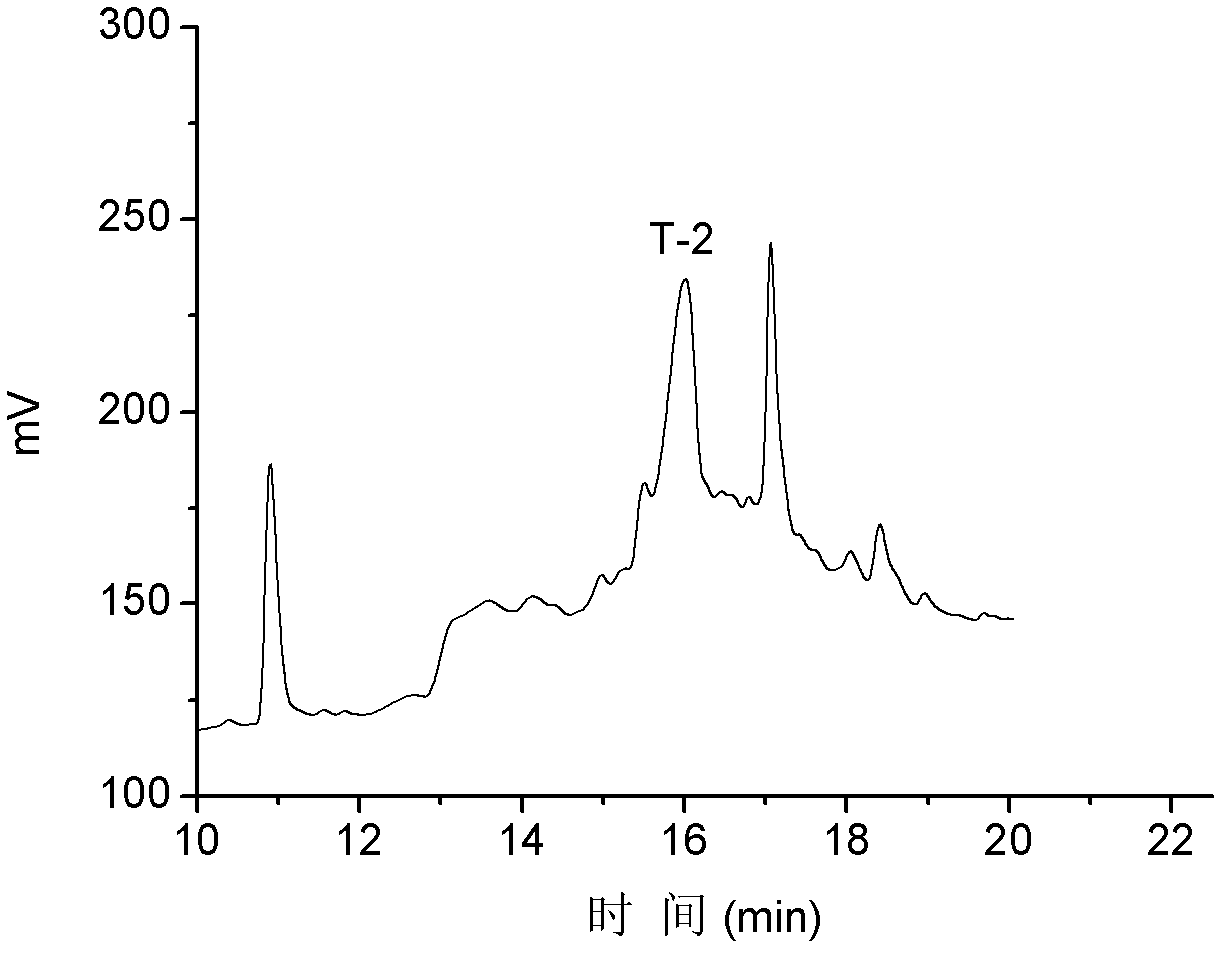
T-2
NH 2 -NCCTRSFCKRIYPDLC*
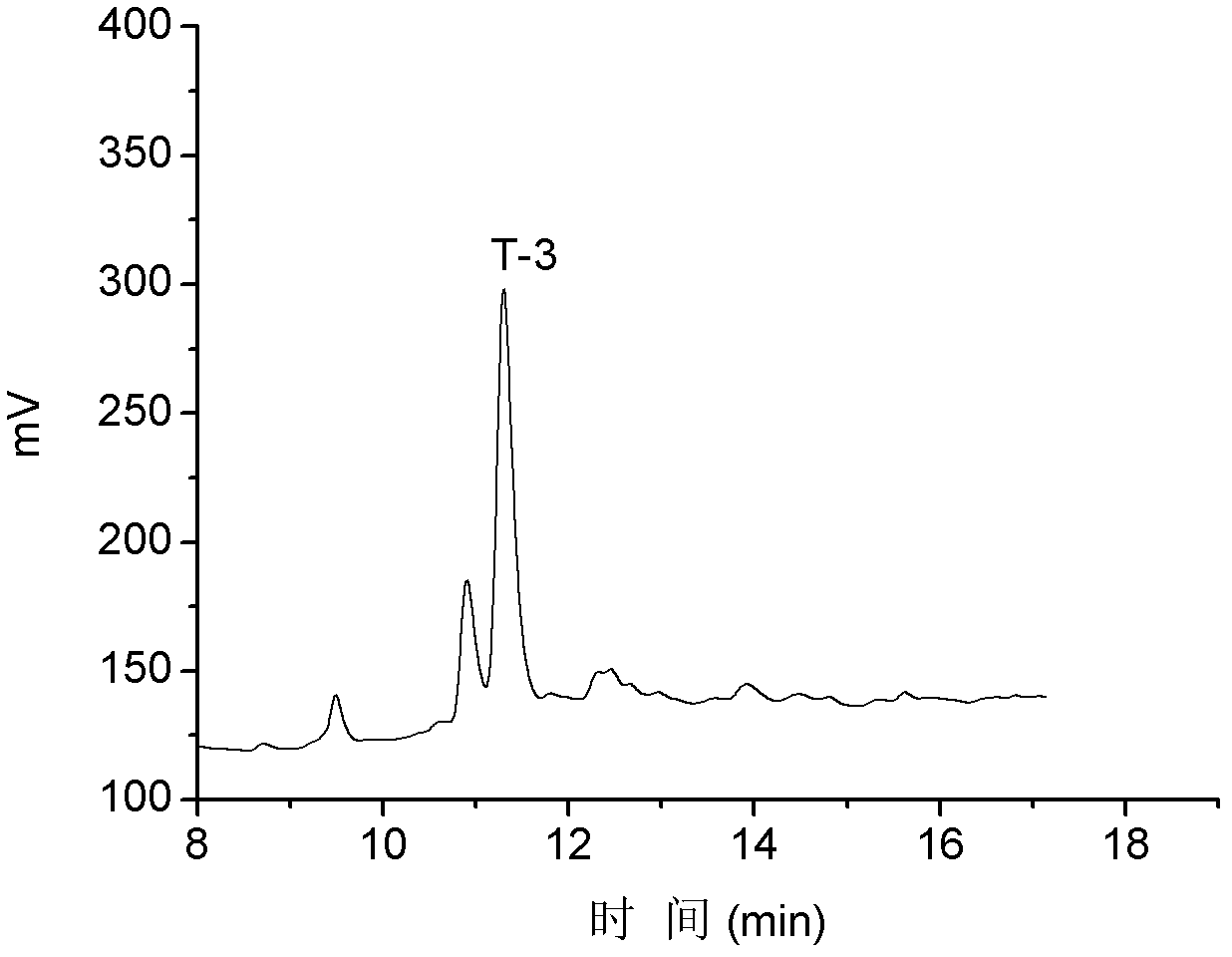
T-3
NH 2 -GCCDIPDCYNKNREQC*
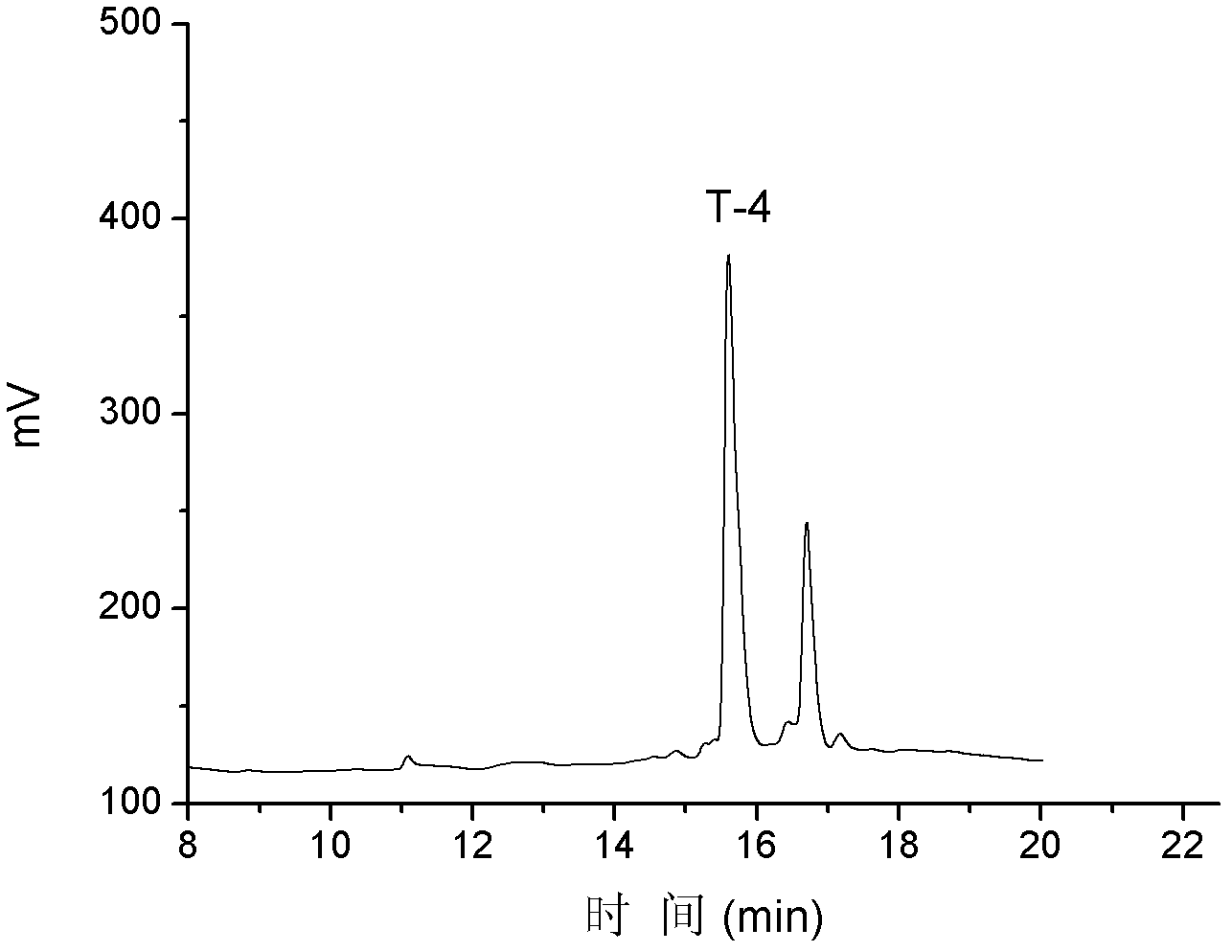
T-4
NH 2 -NCCMFHTCPIDYSRFNC*
T-5
NH 2 -GNCCMFHTCPIDYSRFNC*
T-6
NH 2 -GNCCMFHTCPIDYSRFYC*
T-7
NH 2 -RKCCSNPACNRYNKLC
[0119] D-aspartic acid; E-glutamic acid; I-isoleucine; K-lysine; L-leucine; M-methionine; N-asparagine; Q-glutamine; R- Arginine; S-serine; T-threonine; G-glycine; F-phenylalanine; P-proline; H-histidine; A-alanine; Y-tyrosine. For T-1 and T-7, the disulfide bond connection mode in each polypeptide is C1-C3, C2-C4. * Indicates amidation (CONH 2 ).
[0120] 1. Peptides
[0121] (1) Synthesis of T-1 (artificial synthesis using Fmoc method)
[0122] 1. Synthesis of peptide resin
[0123] Use solid phase synthesis technology. It was carried ...
Embodiment 3
[0173] Example 3 Determination of analgesic activity of α-type Cono polypeptide
[0174] 1. Preparation of α-Cono polypeptide Vc1.1
[0175] α-Cono polypeptide Vc1.1: GCSDPRCNYDHPEICONH 2 .
[0176] 1. Synthesis of peptide resin
[0177] Use solid phase synthesis technology. It was carried out on the 433A peptide synthesizer (ABI American Applied System Biology Inc.). The amino acid used in the synthesis is Fmoc protected amino acid (product of Advanced Chemtech, USA), the resin used is Rink resin (product of Advanced Chemtech, USA), and the reagents used include DCC (Acros), HOBt (product of Advanced Chemtech, USA), NMP (PE company), piperidine (Shanghai Jier Biochemical), methanol (domestic analytical grade) and dichloromethane (domestic analytical grade).
[0178] The side chain protecting groups of Fmoc amino acids are as follows: the side chain protecting groups of His and Cys are trityl (-Trt), and the side chain protecting groups of Arg are 9-phenylfluorenylmethyl (-Pbf) and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com