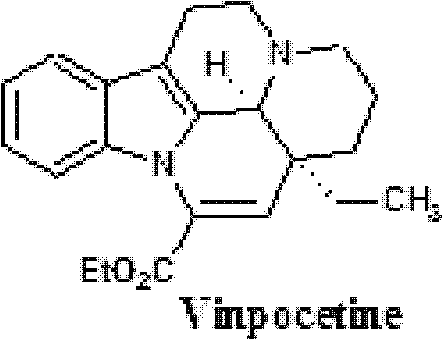
A kind of oral sustained-release solid preparation with vinpocetine as active ingredient
A vinpocetine, slow-release solid technology, applied in the field of medicine, can solve the problems of low patient compliance and frequent drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
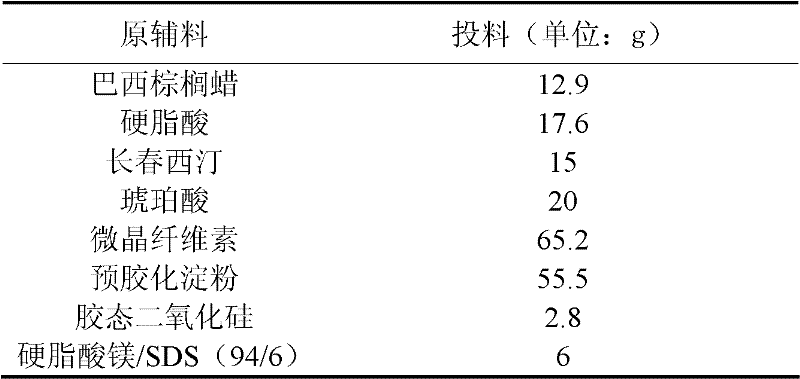
[0034] Embodiment 1 Vinpocetine sustained-release tablet
[0035] Make 1000 Vinpocetine slow-release tablets with the raw materials of following weight ratio
[0036] Extended release partial prescription:
[0037]
[0038] Sustained release part preparation process:
[0039] 1. Pass succinic acid through a 100-mesh sieve, crush vinpocetine, pass through a 100-mesh sieve, and set aside.
[0040] 2. Take carnauba wax, stearic acid, and succinic acid, heat in a water bath at 90°C, stir, and melt, add succinic acid, stir evenly, add vinpocetine, and stir evenly. Stirring was continued, the water bath was removed, and the mass was allowed to cool. A waxy lump was obtained.
[0041] 3. Pulverize the waxy block obtained above, pass through a 60-mesh sieve, add microcrystalline cellulose, pregelatinized starch, and colloidal silicon dioxide, and mix well.
[0042] 4. Add magnesium stearate / SDS (94 / 6) as a lubricant and mix well.
[0043] 5. Prepare 6% Opadry common coating a...
Embodiment 2
[0045] Embodiment 2 Vinpocetine slow-release dry suspension
[0046] Extended release partial prescription:
[0047]
[0048]
[0049] Sustained release part preparation process:
[0050] 1. Pass succinic acid through a 100-mesh sieve, crush vinpocetine, pass through a 100-mesh sieve, and set aside.
[0051] 2. Take carnauba wax, stearic acid, and succinic acid, heat in a water bath at 90°C, stir, and melt, add succinic acid, stir evenly, add vinpocetine, and stir evenly. Stirring was continued, the water bath was removed, and the mass was allowed to cool. A waxy lump was obtained.
[0052] 3. Crush the waxy block obtained above, pass through a 60-mesh sieve, add microcrystalline cellulose, pregelatinized starch, and colloidal silicon dioxide, mix well, add water-based soft materials, extrude and spheronize to make pellets, During the spheronization process, talcum powder is continuously added, and the particle size is 0.5-1.0mm. Sustained-release pellets 1 were obtai...
Embodiment 3
[0069] Embodiment 3: common tablet
[0070] Make 1000 Vinpocetine tablets with the raw materials of following weight ratio
[0071] prescription:
[0072]
[0073] Preparation Process:
[0074] 1. Crush Vinpocetine, pass through a 100-mesh sieve, and set aside.
[0075] 2. Take microcrystalline cellulose and lactose, grind them through a 60-mesh sieve, and set aside.
[0076] 3. Take microcrystalline cellulose, lactose, vinpocetine, and HPMC K4M and mix them uniformly using the method of equal volume increments. Use purified water as a binder to make soft materials, granulate with 24 sieves, dry at 60°C, and control the moisture at 3-5%, granulated with 40 mesh sieve.
[0077] 4. Add magnesium stearate as a lubricant and mix well. Tablet.
[0078] 5. Carry out film coating to above-mentioned plain tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com