Bifonazole vaginal effervescent tablet and its preparation method
A technology of bifonazole and effervescent tablets is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc. Large volume, easy to save, easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
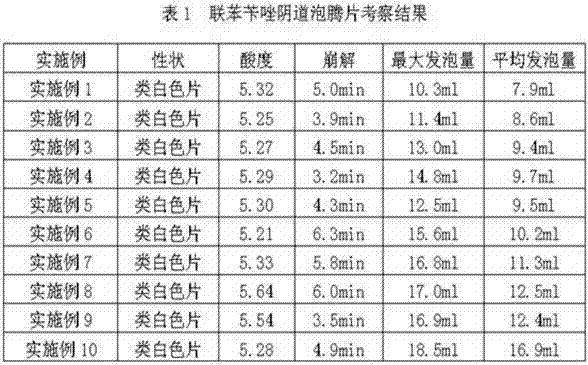
Embodiment 1
[0025] Vaginal effervescent tablets are composed of: 100 parts of bifonazole, 200 parts of sodium bicarbonate, 60 parts of low-substituted hydroxypropyl cellulose, 70 parts of microcrystalline cellulose, 50 parts of lactose, 87 parts of sodium starch glycolate, polyvinyl chloride Ketone K30 10 parts, tartaric acid 215 parts, magnesium stearate 8 parts.
[0026] Preparation method: crush various raw and auxiliary materials separately, pass through a 80-mesh sieve, and set aside; take the prescribed amount of bifonazole, sodium bicarbonate, partly low-substituted hydroxypropyl cellulose, microcrystalline cellulose, lactose, and carboxymethyl starch Mix the sodium evenly, use 5% povidone K30 ethanol solution to make soft material, use 14-mesh nylon sieve to granulate, dry the wet granules at 65°C until the water content is less than 1%, take them out, and use 14-mesh nylon sieve to granulate to obtain Granule A: Mix tartaric acid with the remaining low-substituted hydroxypropyl c...
Embodiment 2
[0028] Vaginal effervescent tablets are composed of: 100 parts of bifonazole, 265 parts of sodium bicarbonate, 70 parts of low-substituted hydroxypropyl cellulose, 100 parts of microcrystalline cellulose, 70 parts of lactose, 60 parts of sodium starch glycolate, hydroxypropyl cellulose 10 parts of methylcellulose, 275 parts of citric acid, 20 parts of polyethylene glycol.
[0029] Preparation method: crush various raw and auxiliary materials separately, pass through a 80-mesh sieve, and set aside; take the prescribed amount of bifonazole, sodium bicarbonate, partly low-substituted hydroxypropyl cellulose, microcrystalline cellulose, lactose, and carboxymethyl starch Mix the sodium evenly, make soft material with 5% hypromellose ethanol solution, granulate with 14-mesh nylon sieve, dry the wet granules at 70°C until the water content is ≤1%, take them out, and granulate with 14-mesh nylon sieve. Granule A is obtained; tartaric acid is mixed with remaining low-substituted hydrox...
Embodiment 3
[0031] Vaginal effervescent tablets are composed of: 80 parts of bifonazole, 290 parts of sodium bicarbonate, 70 parts of low-substituted hydroxypropyl cellulose, 70 parts of microcrystalline cellulose, 60 parts of lactose, 90 parts of sodium starch glycolate, polyvinyl chloride Ketone K30 24 parts, tartaric acid 300 parts, polyethylene glycol 8 parts, sodium lauryl sulfate 2 parts.
[0032] Preparation method: crush various raw and auxiliary materials separately, pass through a 80-mesh sieve, and set aside; take the prescribed amount of bifonazole, sodium bicarbonate, partly low-substituted hydroxypropyl cellulose, microcrystalline cellulose, lactose, and carboxymethyl starch Mix the sodium evenly, use 10% povidone K30 ethanol solution to make soft material, use 14-mesh nylon sieve to granulate, dry the wet granules at 70°C until the water content is less than 1%, take them out, and use 14-mesh nylon sieve to granulate to obtain Granule A: Mix tartaric acid with the remaining...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com