Pharmaceutical applications of hq-091212
A drug and pharmaceutical technology, applied in the field of drug application of HQ-091212, can solve the problems of limited application, severe side effects, and unclear anti-tumor spectrum, and achieve the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0035] The following examples illustrate several representative dosage forms containing HQ-091212 suitable for human use.
[0036]
[0037]
[0038]
[0039]
[0040]
[0041]
[0042]
[0043] Example of Pharmacology and Pharmacodynamics Evaluation
experiment Embodiment 1
[0045] Inhibitory effect of HQ-091212 on the proliferation of human tumor cells in vitro
[0046] method
[0047] Tumor cells were cultured in RPMI 1640 or DMEM medium (Gibco) containing 10% fetal bovine serum at 37°C, 5% CO 2. Tumor cells were inoculated in 96-well plates. After 24 hours, HQ-091212 prepared with dimethyl sulfoxide was added to make the final concentration of HQ-091212 in the medium 0.0001-100 μM; The final concentration does not exceed 0.1%. After HQ-091212 treatment for 72 hours, the culture medium was discarded and the cells were fixed with cold trichloroacetic acid. Then stained with sulforhodamine B (Sulforhodamine B, SRB) solution. After washing away the unbound SRB, use Tris to dissolve the SRB bound to the protein, measure the OD value with a microplate reader at a wavelength of 520nm, and calculate the cell growth inhibition rate with the following formula:
[0048] Inhibition rate = (OD value control well - OD value administration well) / OD val...
experiment Embodiment 2
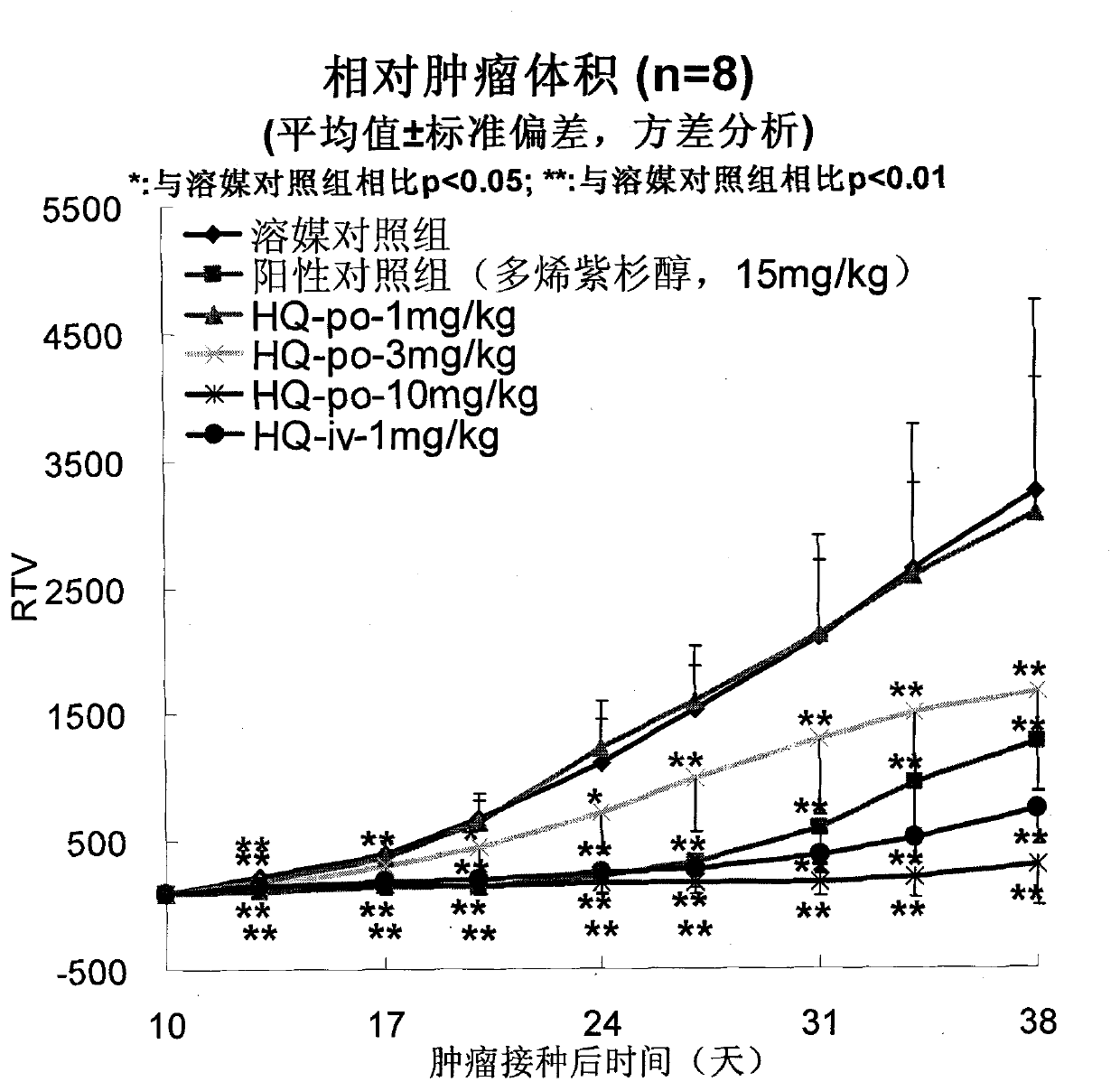
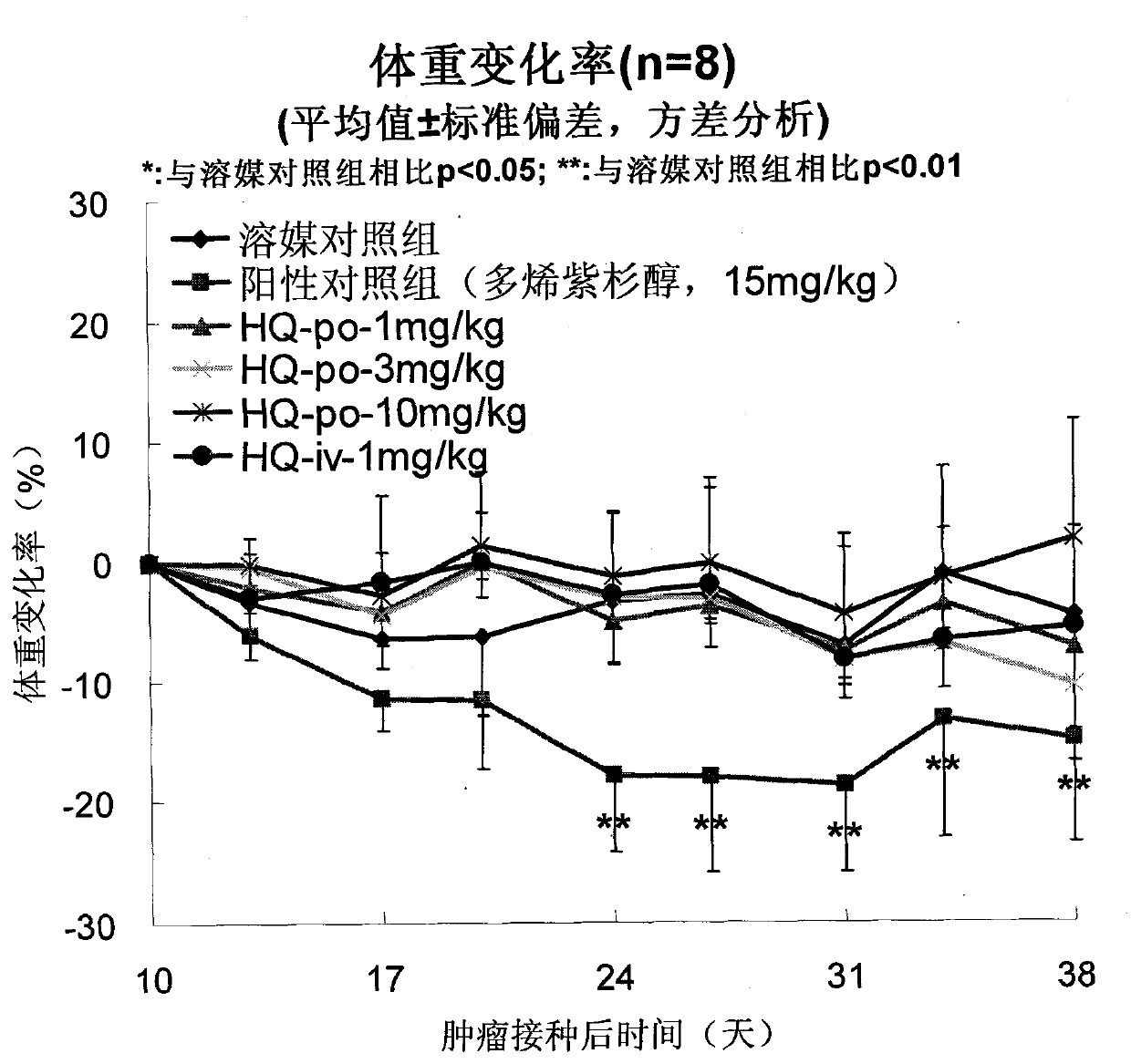
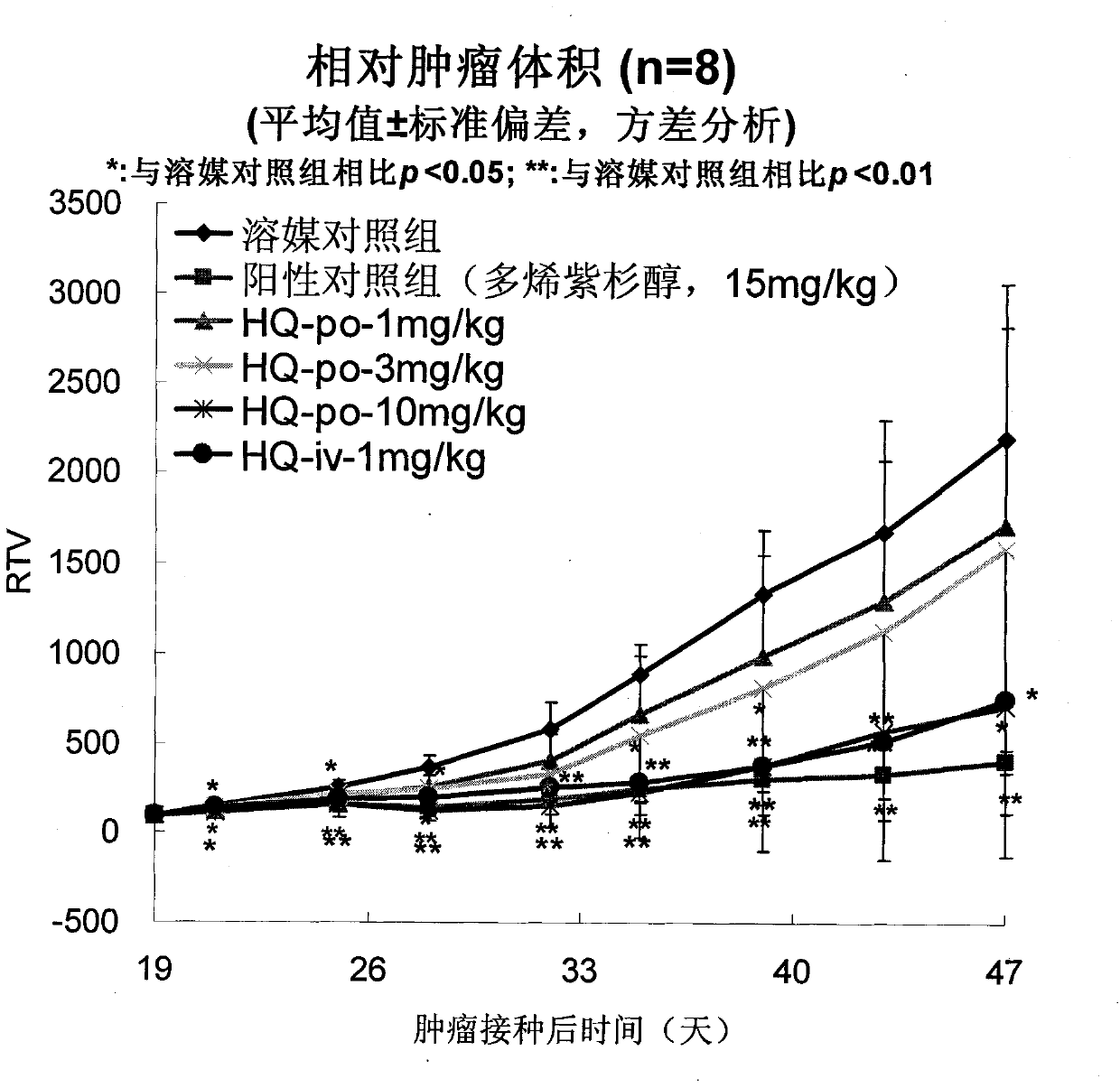
[0055] Efficacy of oral administration of HQ-091212 on human prostate cancer PC-3 xenografted tumor in nude mice
[0056] method
[0057] Human prostate cancer PC-3 was purchased from American Type Culture Collection (ATCC).
[0058] BALB / c nude mice, ♂, 6 weeks old, were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences. Breeding environment: SPF grade.
[0059] Nude mice were subcutaneously inoculated with PC-3 human prostate cancer cell line, the inoculation volume was 5×10 per mouse 6 cells, and then cut the nude mouse tumor tissue into 2mm 3 Square small pieces, implanted subcutaneously in 5-6 week-old nude mice, until the tumor grows to at least 100mm 3 Afterwards, the animals were randomly divided into groups HQ-091212: oral administration of 1, 3, 10 mg / kg, once a day, for 3 consecutive weeks; positive control docetaxel 15 mg / kg, intravenous injection, once a week, a total of 3 weeks . The tumor volume was measured twice a week, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com