Polyethylene glycol derivative of enkephalin analogue
A technology of PEGylation and polyethylene glycol, applied in the field of medicine, can solve the problems of increasing the cost of synthesis and production, difficult control of reaction conditions, affecting the yield and purity of modified products, and achieving high yield and purity, and the reaction Conditional effects that are easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Preparation of precursor-C-terminal cysteine MEK (MEK-Cys-NH 2 ) and C-terminal cysteine DADAE (DADAE-Cys-NH 2 ):
[0074] The amino acids adopted include Fmoc-Tyr(tBu)-OH, Fmoc-Gly-OH, Fmoc-Phe-OH, Fmoc-Met-OH, Fmoc-D-Ala-OH, Fmoc-Cys(Trt)-OH; wherein tBu is tert-butyl, and Trt is trityl. Synthesis of polypeptide sequences was performed manually. The solid-phase polypeptide synthesis glass reactor is custom-made, and the upper and lower chambers are separated by G2 filter plates, which are treated with silane reagents before use.
[0075] Steps:
[0076] 1. MEK-Cys-NH 2 and DADAE-Cys-NH 2 Synthesis:
[0077] (1) Rink Amide-AM resin, 0.160g (0.1mmol), swell in DMF for 10min, 20% piperidine / DMF1×10ml, remove Fmoc protection for 30min;
[0078] (2)CH 2 Cl 2 2×10ml, wash for 1min, including bottle cap;
[0079] (3) DMF1×10ml, wash for 1min;
[0080] (4)CH 3 OH1×10ml, wash for 1min;
[0081] (5) DMF1×10ml, wash for 1min;
[0082] (6)CH 3 OH1×10...
Embodiment 2
[0115] Example 2 Reacting enkephalin analogs with polyethylene glycol to synthesize PEGylated derivatives of enkephalin analogs:
[0116] Steps:
[0117] 1. mPEG 5000 -MAL generation
[0118] (1) Weigh mPEG 5000 -OH40.0g (8mmol), dissolved in 100ml CH 2 Cl 2 15ml of triethylamine and 19g of p-toluenesulfonyl chloride were added after stirring to dissolve, and the reaction was stirred at room temperature for about 6 hours. After the reaction was complete as detected by TLC, the solvent was removed by rotary evaporation, and 100ml of anhydrous ether was added to precipitate a solid to obtain mPEG 5000 -OTs.
[0119] (2) For the obtained mPEG 5000 -OTs were purified. Crude mPEG 5000 -OTs is dissolved in water, first use a separatory funnel to extract p-toluenesulfonyl chloride with ether, discard the ether layer, and then use CH 2 Cl 2 Extraction of mPEG in water 5000 -OTs, the aqueous layer was discarded, dried over anhydrous magnesium sulfate, and CH was removed by r...
Embodiment 3
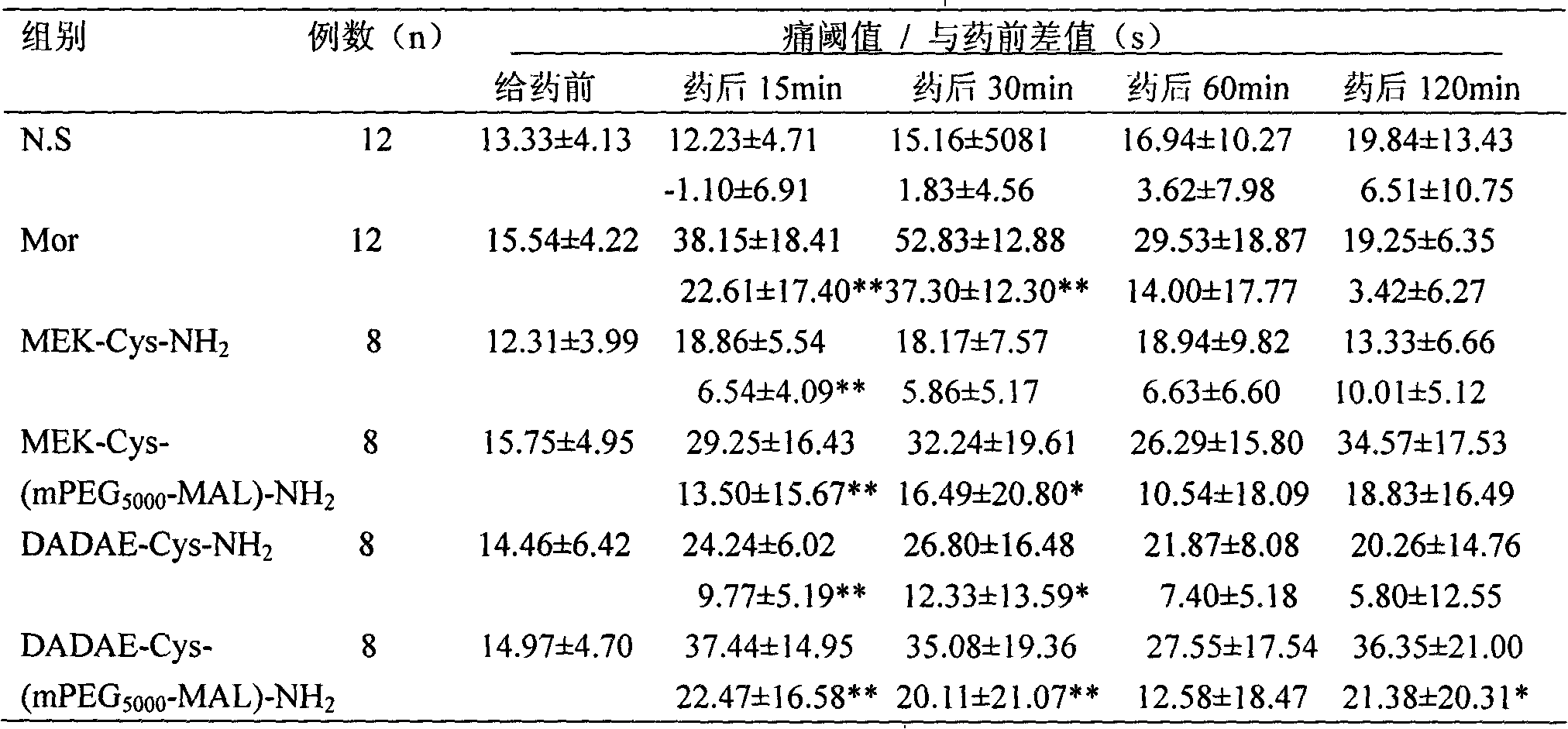
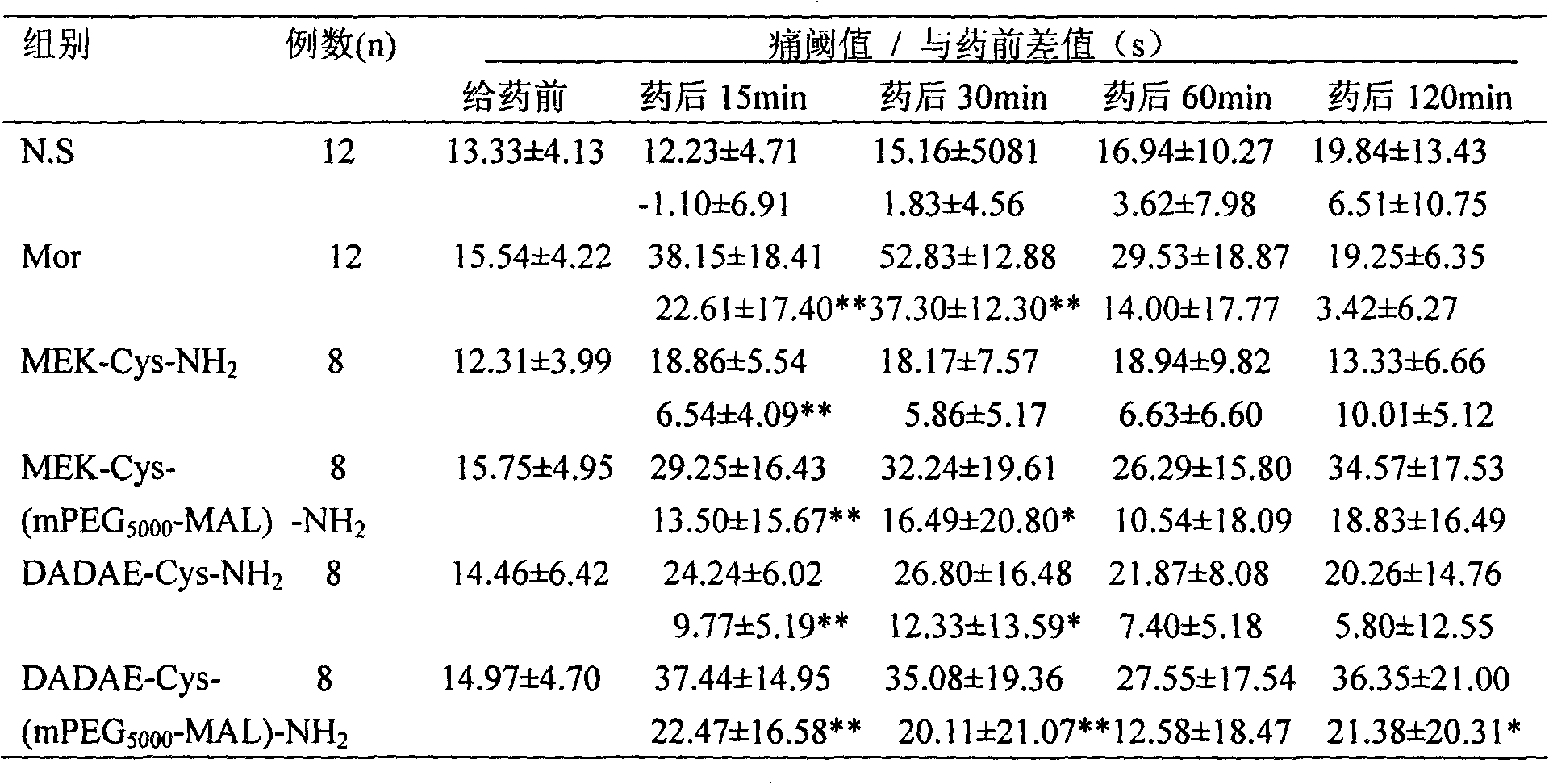
[0134] Example 3 Pharmacodynamic experiment - detection of analgesic pharmacological activity of synthetic peptides:
[0135] In this example, the analgesic activity of four enkephalin analogues and polyethylene glycol modifications was determined, specifically including: MEK-Cys-NH 2 , DADAE-Cys-NH 2 , MEK-Cys (mPEG 5000 -MAL)-NH 2 , DADAE-Cys (mPEG 5000 -MAL)-NH 2 (Preparation of Example 1 and 2 of the present invention). When used, they were dissolved in normal saline according to the equimolar concentration of the control drug morphine, sterilized by filtration through a 0.45 μm filter membrane, and injected into the tail vein or lateral ventricle.
[0136] The control drug in this embodiment is morphine hydrochloride (Dexamethasone, Mor; produced by Northeast Sixth Pharmaceutical Factory, batch number: 001009, specification 10 mg / ml). When used, the clinical equivalent dose (10 mg / kg) converted from animal to human body weight is diluted with sterile normal saline, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com