Process method for preparing methoxycephems intermediate 7-MAC (7-methoxycephalosporin)
A technology of methoxycephalosporin and process method, which is applied in the field of preparation of methoxycephalosporin intermediate 7-MAC, which can solve the problems of difficult precise control, fluctuation of content and light transmittance, many changing factors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
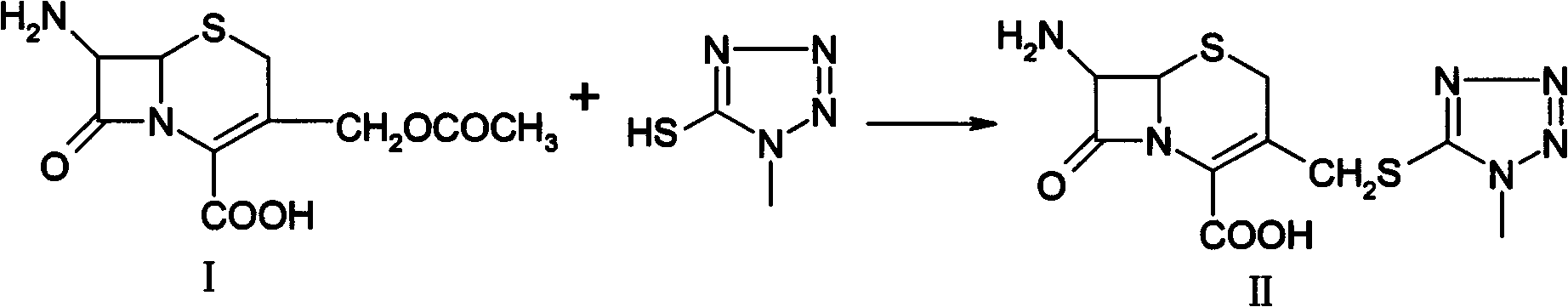
[0034] (1) Synthesis of formula II7-TMAC: In a 1000ml four-necked reaction flask, add 27.2 grams of 7-ACA, 12 grams of 1-methyl-5-mercaptotetrazolium, and 140 grams of acetonitrile, stir well, and place at 30°C to 40°C, add 10 grams of boron trifluoride ether and 25 grams of methanesulfonic acid dropwise, continue the heat preservation reaction at 30-40°C for 1 hour after the drop, cool the reaction solution to 0°C to 5°C after the reaction, add 600°C of purified water dropwise After adding water, add dropwise 30% liquid caustic soda to adjust the pH value to 4.0, control the temperature below 10°C, and stir for 2 hours to crystallize. Then filter, and the filter cake is beaten and washed with water once more. Vacuum-dried at 40°C until the moisture content was less than 0.5%, to obtain 30.6 g of the product.
[0035] (2) Synthesis of formula III intermediate:
[0036] A. Preparation of sulfur bromide reagents:
[0037] ①In a 250ml four-necked bottle, add 8.1 grams of dimet...
Embodiment 2
[0056] (1) The synthesis of 7-TMCA is the same as in Example 1.
[0057] (2) Synthesis of formula III intermediate:
[0058] A. The preparation of methyl sulfur bromide is the same as in Example 1.
[0059] B. The preparation of diphenyldiazomethane is the same as the method in Example 1 1.
[0060] C. Synthesis of the intermediate of formula III: 1000ml four-necked reaction flask with reflux condenser, put 25.5 grams of 7-TMCA into it, stir 180 grams of methylene chloride for 10 minutes, add 32 grams of hexamethyldisilamine (HMDS for short) dropwise , After dropping, react at 25°C for 2 hours, cool to 10°C and add 5 grams of propylene oxide, then add dropwise the methyl sulfur bromide solution obtained in step A, and react at 35°C for 2 hours after dropping. Then add the diphenyldiazomethane solution obtained by the manganese dioxide method in Step B, and react at 35° C. for 3 hours. Add 200 g of water, stir and separate the layers. The organic layer is concentrated under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| translucency | aaaaa | aaaaa |
| relative humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com