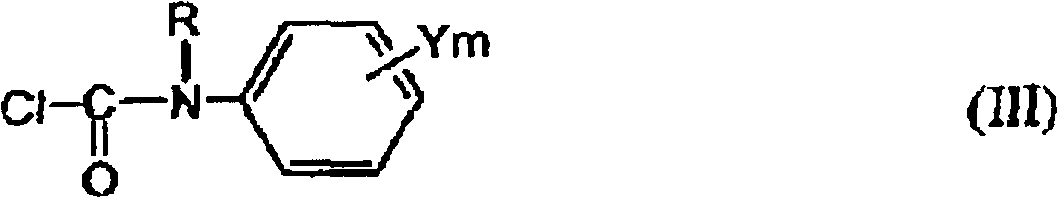Preparation method of 1-substituted-4-carbamoyl-1,2,4-triazol-5-one derivatives
A technology for carbamoyl and derivatives, which is applied in the field of preparation of 1-substituted-4-carbamoyl-1,2,4-triazol-5-one derivatives, and can solve problems such as complicated operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085]
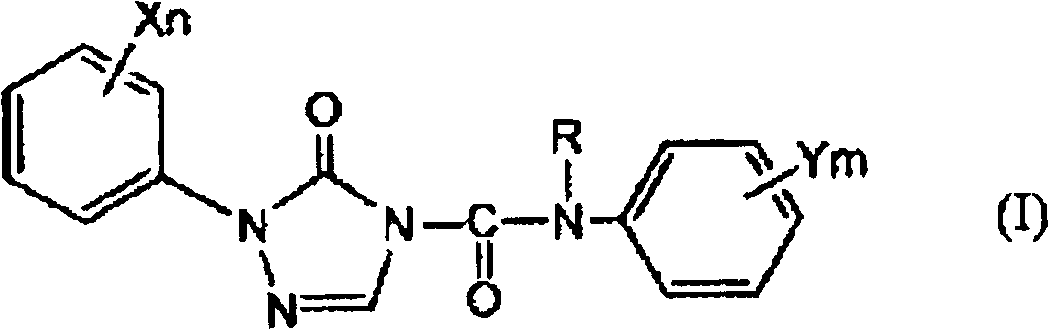
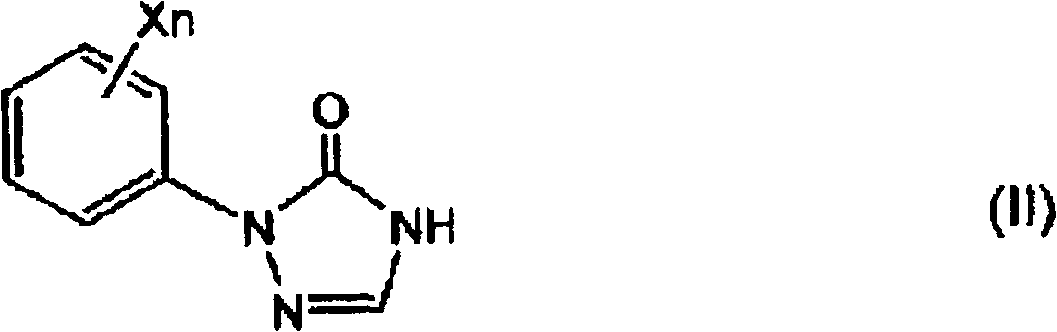
[0086] In the present invention, as described above, in order to obtain the 1-substituted-4-carbamoyl-1,2,4-triazol-5-one derivative (I) as the target compound, step 2 is carried out after step 1, thereby First, the step 1 for obtaining the target 1-substituted-4-carbamoyl-1,2,4-triazol-5-one derivative (I) will be described.
[0087]
[0088] In step 1 of the present invention, in the selected from quaternary ammonium salt and In the presence of at least one phase transfer catalyst (B) of a salt, the 1-substituted-1,2,4-triazole-5- Ketone derivative and (b) alkali metal hydroxide (A) react, obtain the alkali metal salt ( During IIa), the by-produced water is removed by azeotropic distillation together with the solvent (C) and discharged to the outside of the system.
[0089] (Conditions of temperature and pressure and time)
[0090] The reaction in step 1 can be carried out at normal temperature (15-25° C.) or heating {the temperature depends on the type of s...
Synthetic example 1
[0140] Synthesis of 1-(2,4-dichlorophenyl)-1,2,4-triazol-5-one
[0141] Analysis of the reaction and purity was carried out by high-performance liquid chromatography (HPLC) under the following conditions.
[0142] Device: Shimadzu Corporation HPLC system, UV detector: SPD-10AVP, pump: LC-10ATVP, column oven: CTO-10ASVP, column: Japan GL Science (GL Science) Co., Ltd. Inertsil ODS-3 (column The particle size of filler: 5 μm, column inner diameter: 4.6mm, column length: 250mm), UV detection wavelength: 254nm, mobile layer solvent volume ratio and flow rate: acetonitrile: distilled water=60: 40, 1.0ml / min, (as the first 1 When analyzing the hydrazone of the intermediate product), methanol:distilled water=10:3, 0.5ml / min, (when analyzing the triazolinone and the target compound as the second intermediate product), use Japan Yamato (Yamato) The melting point of the compound was measured with a melting point analyzer MP-21 manufactured by Science Co., Ltd., and the mass spectrum w...
Synthetic example 2
[0148] Synthesis of N-isopropyl-N-2,4-difluorophenylcarbamoyl chloride
[0149] Analysis of reaction and purity was carried out by gas chromatography under the following conditions.
[0150] Device: Shimadzu Corporation GC-14B, column: G-100 (column length 20m, film thickness 0.5μm, ID 1.2mm), analysis conditions: 80°C (initial), 20°C / min (heating to 280°C (final)), detector: TCD (300° C.), carrier gas: helium (20 ml / min), and mass spectrometry of the compound was measured with a mass spectrometer SUN-200 manufactured by JEOL Ltd.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com