Recombination expression of cynoglossus semilaevis antibacterial peptide hepcidin and application thereof
A technique of semi-smooth tongue sole and antimicrobial peptide, which is applied in the field of recombinant expression of the semi-smooth tongue sole antimicrobial peptide hepcidin, can solve the problems of high cost, complicated synthetic process of antimicrobial peptide, difficult mass production and the like, and achieves the effect of solving the harm of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Construction of embodiment 1 recombinant expression vector, identification and expression of antimicrobial peptide, purification
[0019] 1. Construction and identification of the recombinant plasmid: According to the mature peptide cDNA sequence (SEQ ID NO: 2) of the antibacterial peptide hepcidin of half-smooth tongue sole, it was modified according to the codon preference of Escherichia coli, and a pair of specific primers P1 and P2 were used to introduce The restriction endonuclease sites EcoRI and NotI sites are used to amplify the mature peptide of the antimicrobial peptide of half-smooth tongue sole, and the primer sequence is:
[0020] P1: 5′-GGAATTCCGTGTGAAGCGC-3′
[0021] P2: 5'-TATGCGGCCGCTTAGTATTTACAAC-3';
[0022] The PCR reaction volume was 25ul, and the amplification conditions were: 94°C for 5min; 94°C for 30s, 55°C for 30s, and 72°C for a total of 28 cycles; 72°C for 7min. After the PCR product was detected by agarose electrophoresis, the product was ...
Embodiment 2
[0029] Antibacterial activity assay of embodiment 2 recombinant protein
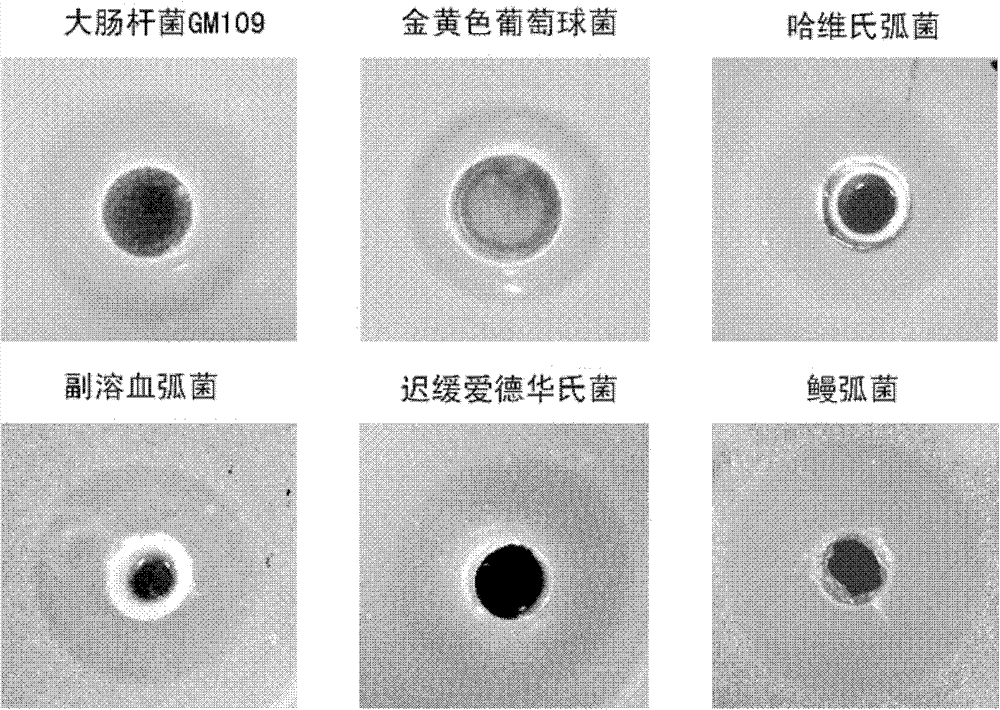
[0030] Use the agar hole diffusion method to detect the antibacterial activity of the antibacterial peptide hepcidin recombinant protein of half-smooth tongue sole: 200uL of the suspension of the test bacteria in the logarithmic growth phase (OD600=0.2~0.3) was mixed with LB solid medium (or seawater) at 55°C Base 2216E) 25mL was mixed evenly and plated. After it was solidified, a hole was punched with an Oxford cup (diameter 8mm), and about 1.5uM of purified recombinant antimicrobial peptide was added dropwise to the hole, and incubated at 37°C (or 28°C) for 8-12h. The same volume of PBS was used as a negative control, and the diameter of the inhibition zone was measured on the second day.
[0031] The tested bacteria include common bacteria: Escherichia coli GM109 (E.coli), Staphylococcus aureu (Staphylococcus aureu), and major marine pathogens: Vibrio harveyi (Vibrio harveyi), Vibrio parahaemolyticus ...
Embodiment 3
[0032] Example 3: Application of recombinantly produced antimicrobial peptides in the preparation of antibacterial products
[0033] Escherichia coli containing the fusion protein expression vector can be produced as a powder by methods such as microfiltration, ultrafiltration, spray drying, and lyophilization after conventional culture, induced expression, and purification methods provided by the present invention to obtain semi-finished products of antimicrobial peptides, or The liquid preparation is obtained after being refined and purified by biochemical methods. The obtained antimicrobial peptide semi-finished products or veterinary drug-grade finished products can be directly used as antibacterial drugs in conventional dosages for the prevention and treatment of aquatic organisms or other farmed poultry diseases.
[0034] Select fish meal, bone meal, corn meal, bran, bran, bean cake, etc. as the basic feed for aquatic organisms or farmed livestock in a conventional manne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com