Biotransformation and purification method of 4-(2,3,5,6-tetramethylpyrazine-1-group)-4'-demethylepipodophyllotoxin
A technology of removing epipodophyllum and tetramethylpyrazine, applied in the field of biotransformation of podophyllin-like compounds, can solve the problems of high price, high production cost, small scale, etc., and achieve simple operation, short cycle and easy cultivation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
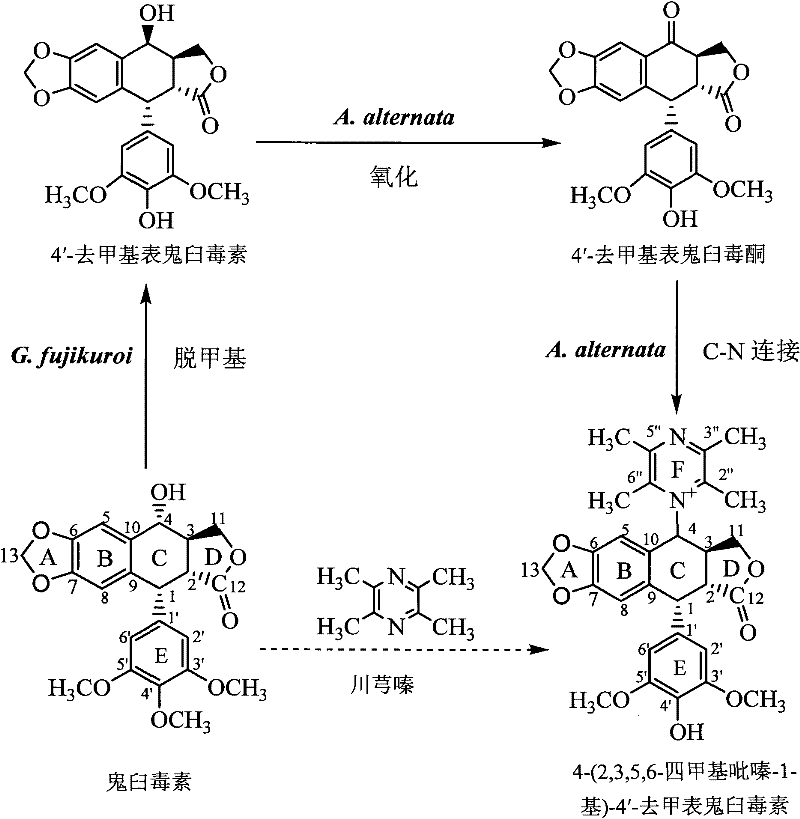
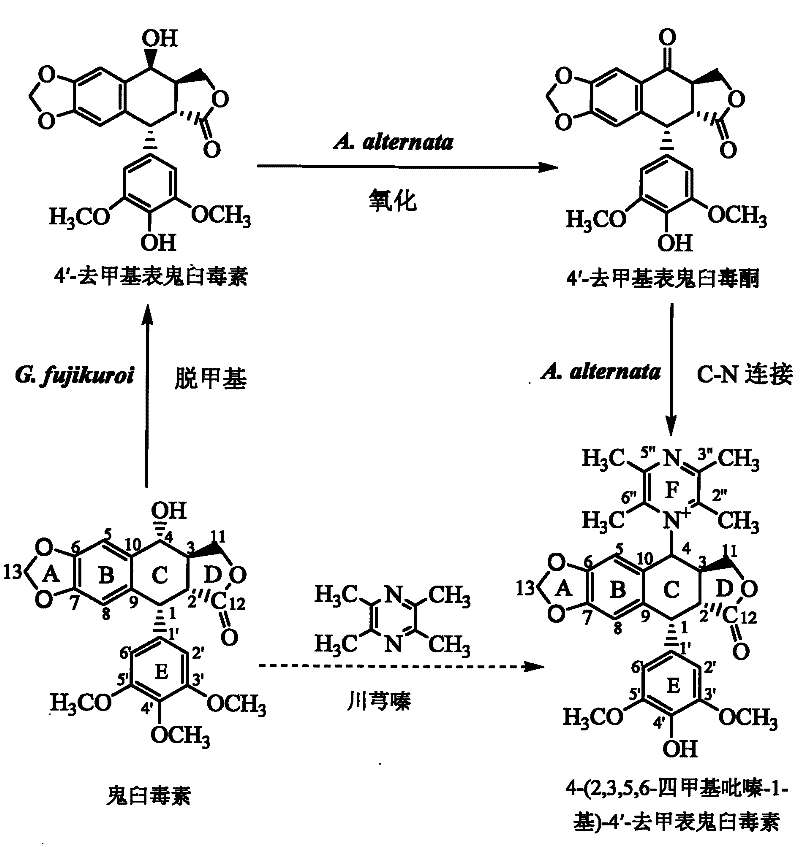
[0072] 1. In the first stage, podophyllotoxin is converted into 4′-desmethyl epipodophyllotoxin:
[0073] 1), slant strain cultivation: the formula of the slant strain culture medium adopted in the present embodiment is (nutritional PDA slant): glucose 30 grams / liter, magnesium sulfate 0.5 grams / liter, potassium dihydrogen phosphate 1.5 grams / liter, Thiamine hydrochloride 0.1 g / L, agar 20 g / L, potato extract 1.0 L; the preparation method of potato dipping juice: 200g fresh potatoes are peeled and cut into square pieces with a side length of about 1cm, and the cut pieces Put it in an enamel tank with 1L of deionized water, boil for 15 minutes, and when the potatoes are soft and not loose, filter them with eight layers of gauze, take the filtrate and dilute it to 1L again, and the obtained solution is used to prepare PDA medium.
[0074] Gibberella Fujikura SH-f13 was inoculated on the PDA slant by the two-point method with an inoculation needle, and the culture conditions were ...
Embodiment 24
[0097] Example 24-(2,3,5,6-tetramethylpyrazin-1-yl)-4'-norepipodophyllotoxin separation and purification
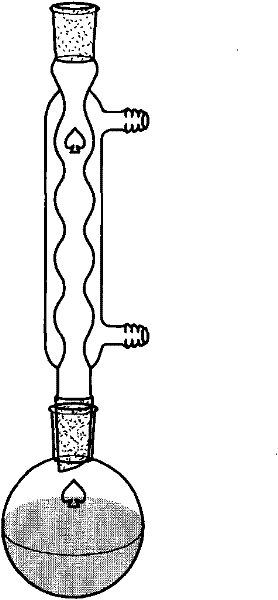
[0098] (1), prepare the sample to be separated: take the Alternaria tenospora mycelium obtained in Example 1 and place it in a 60°C oven for 4 days, and after the mycelium is completely dry, grind it into a powder sample with a pulverizer, and take 200 grams This sample is in the round bottom flask of 2 liters and adds a small amount of broken ceramic pieces, adds 1 liter of chloroform, the upper end of the round bottom flask is connected with the reflux extractor, and the upper end of the extractor is connected with the condensing pipe cold water reflux ( figure 2 ). Heating to boiling and maintaining for 2 hours was completed as one time, and the extraction was refluxed three times. The filtered chloroform was rotary evaporated, and the sample was vacuum-dried, and 5 ml of chloroform was taken to reconstitute the dried sample, and stored in a refrigerator at 4°C as my...
Embodiment 34
[0102] Example 34-(2,3,5,6-tetramethylpyrazin-1-yl)-4'-norepipodophyllotoxin content determination
[0103] (1), the fermentation broth obtained in Example 1 was centrifuged (10000 rpm, 20 minutes, 4°C), and 2 ml of supernatant was used for 4-(2,3,5,6-tetramethylpyridine Determination of oxazin-1-yl)-4'-norepipodophyllotoxin; 4 ml of acetonitrile was added to 2 ml of fermentation broth, and centrifuged (13000 rpm, 20 minutes); the supernatant was passed through a 0.45 micron needle Filter through a filter membrane and store in a refrigerator at 4°C as a test sample of the fermentation broth.
[0104] (2), take the fresh mycelium obtained in Example 1 and dry it at 60°C, grind the dried mycelium to 200 mesh powder, take 100 grams of mycelium powder in a 2-liter round bottom flask and add A small amount of broken ceramic pieces, add 1 liter of chloroform, the upper end of the round bottom flask is connected to the reflux extractor, and the upper end of the extractor is connecte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com