Preparation method of cyaniding-3-O-glucoside chloride
A technology of glucoside and cornflower, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., to avoid the use of macroporous resins, simple steps, and safe reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
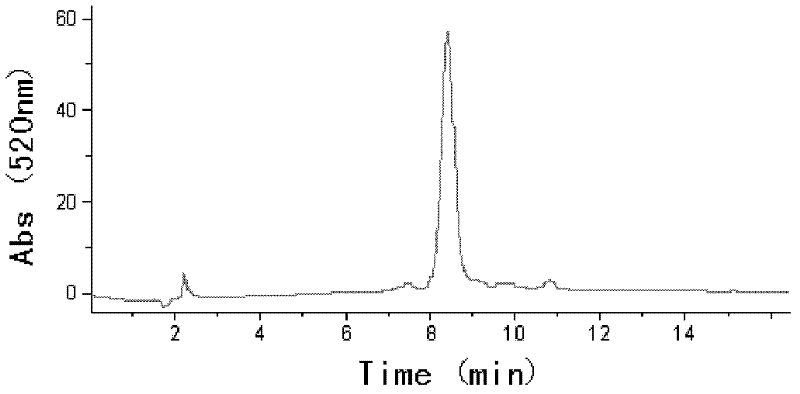
[0028] The raw material is black soybean skin, and the total anthocyanin content is 2.04% after determination, wherein the cyanidin-3-O-glucoside content accounts for more than 90% of the total anthocyanin content (that is, 100 grams of dry black soybean skin contain total anthocyanin Su 2.04g, which contains about 1.83 grams of cyanidin-3-O-glucoside). figure 1 It is the high performance liquid chromatogram (520nm) of total anthocyanins in this black soybean hull raw material.
[0029] Take 1000g of the above-mentioned black soybean hull raw material, use 15L of 70% ethanol acid aqueous solution (adjusted to pH=2.5 with 1mol / L hydrochloric acid in advance) at about 50°C for 3 extractions, each time for 1 hour. The three extracts were filtered and combined through a 100-mesh sieve, concentrated by rotary evaporation (vacuum degree 0.08MPa) at 55°C, concentrated to about 1 / 8 of the original volume, and then spray-dried (air inlet temperature 120°C, air outlet temperature 80 ℃)...
Embodiment 2
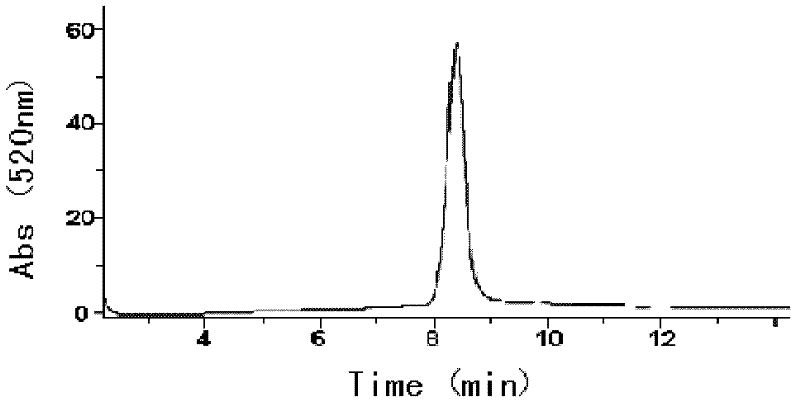
[0034] The raw material is dried purple corn husk pulverized matter, and its total anthocyanin content is 3.49% after determination, wherein the cyanidin-3-O-glucoside content accounts for about 40% of the total anthocyanin content (that is, 100 grams of dried purple corn Containing 3.49g of anthocyanins in the pulverized corn bracts, wherein the content of cyanidin-3-O-glucoside is about 1.40g), the high-performance liquid chromatography of this raw material is shown in image 3 (520nm).
[0035] Take 1000g of crushed purple corn and use 12L of 50% ethanol (pH=3.0) acid aqueous solution to extract twice at about 50°C for 1 hour each time. The two extracts were combined and passed through a 100-mesh sieve, concentrated by rotary evaporation at about 55°C (vacuum degree 0.075MPa) to about 1 / 10 of the original volume, and then spray-dried (air inlet temperature 110°C, air outlet temperature 80°C) to obtain Powdered anthocyanin crude extract 290g.
[0036] The crude extract is ...
Embodiment 3
[0040] The raw material is fresh purple cowpea, the total anthocyanin content is 0.057%, wherein the cyanidin-3-O-glucoside content accounts for more than 93% of the total anthocyanin content (that is, 10000 grams of fresh purple cowpea contains total flower cyanidin 5.7g, which contains about 5.3g of cyanidin-3-O-glucoside).
[0041] Weigh 10Kg of fresh purple cowpea, crush it and put it into an extraction tank, add 150L of 60% ethanol (pH=3) acid aqueous solution to extract twice at 55°C, each time for 1 hour. The two extracts were combined and passed through a 120-mesh sieve, concentrated by rotary evaporation (vacuum degree 0.08MPa) at 50°C to about 1 / 20 of the original volume, and then spray-dried (air inlet temperature 110°C, air outlet temperature 80°C) to obtain powder Anthocyanin crude extract 450g.
[0042] The above-mentioned crude extract is dissolved in double distilled water at a ratio of 1:75 by weight to volume (g / mL), and subjected to ultrafiltration, using a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com