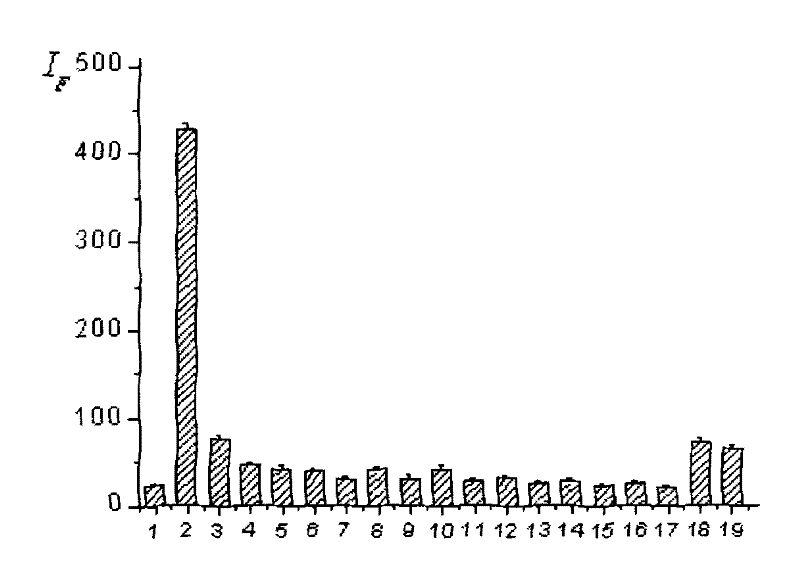
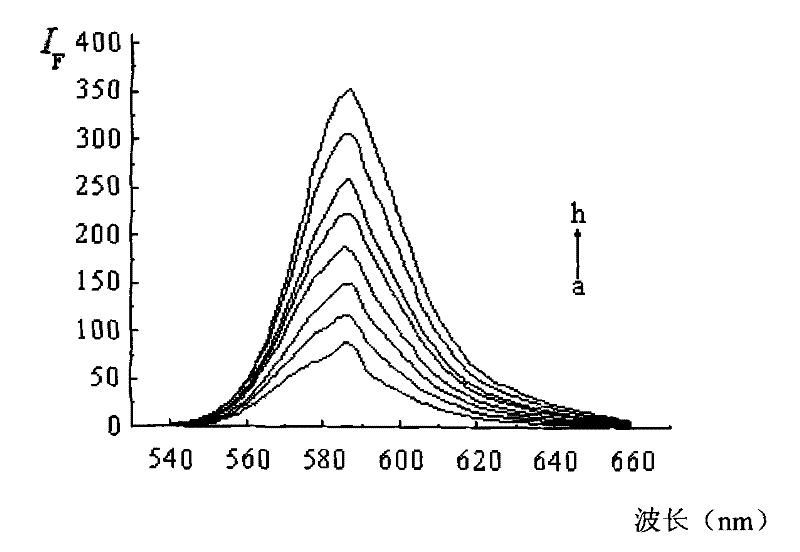
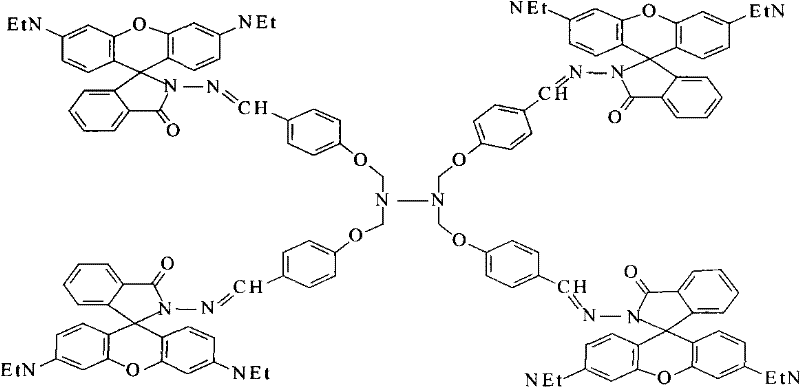
Dendritic fluorescent chemical sensor, and its preparation method and application
A chemical sensor, dendritic technology, applied in the field of fluorescent chemical sensor and preparation, to achieve the effects of fast sensing, easy control of reaction conditions, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] ①Synthesis of rhodamine B hydrazide [RBH]: Add 2.4g rhodamine B to a 100mL round bottom flask, dissolve it completely with 50mL absolute ethanol, pipette 6.0mL of 85% hydrazine hydrate, and add it dropwise to the round bottom In the flask, shake vigorously at room temperature. The mixture was heated and stirred under reflux for 2 h, the solvent ethanol was evaporated, cooled to room temperature, and allowed to stand for 30 min, and then dissolved in 50 mL of 1 mol·L -1 HCl was dissolved in the mixture to obtain a dark red clear solution, and about 70mL of 1mol·L -1 NaOH, until the pH of the solution is about 7, shake, stand still, a brick red precipitate precipitates, filter under reduced pressure, wash with distilled water 10 times, and dry to obtain rhodamine B hydrazide. Stored in a desiccator for later use, the yield is 75%.
[0021] ② Synthesis of tetragonal aldehyde-based compounds: Dissolve hydrazine hydrate [2.9mL, 85%, 0.05mol] in 10mL ethanol, stir well in a...
Embodiment 2
[0024] ①Synthesis of rhodamine B hydrazide [RBH]: Add 2.4g of rhodamine B into a 100mL round bottom flask and completely dissolve it in 40mL of absolute ethanol. Pipette 6.0 mL of 85% hydrazine hydrate, add it dropwise into a round bottom flask, and vibrate vigorously at room temperature. The mixture was heated and stirred at reflux for 2h. The solvent ethanol was evaporated, cooled to room temperature, and allowed to stand for 30 min. Use 50mL 1mol·L -1 HCl was dissolved in the mixture to give a dark red clear solution. Then slowly add about 70mL 1mol·L -1NaOH, until the pH of the solution is about 7, shake, stand still, a brick red precipitate precipitates, filter under reduced pressure, wash with distilled water 10 times, and dry to obtain rhodamine B hydrazide. Stored in a desiccator for later use, the yield is 75%.
[0025] ②Synthesis of tetragonal aldehyde-based compounds: Dissolve hydrazine hydrate [2.9mL, 85%, 0.05mol] in 10mL ethanol, stir well in a three-necked ...
Embodiment 3
[0028] ①Synthesis of tetragonal aldehyde-based compounds: Dissolve hydrazine hydrate [2.9mL, 85%, 0.05mol] in 10mL ethanol, stir well in a three-necked flask, add 28g [0.23mol) p-hydroxybenzaldehyde and dissolve it in 100mL ethanol After the addition, the two were mixed evenly, refluxed at 100°C for 1.2h, cooled and filtered, and the crude product was recrystallized with absolute ethanol to obtain a light yellow product of tetragonal aldehyde compound with a yield of 75%.
[0029] ②Synthesis of dendritic fluorescent chemical sensor: Under the protection of argon, take 0.4g [0.08mol] tetragonal aldehyde compound and dissolve it in 30mL ethanol in a three-necked flask, and add 1.72g [0.36mol] rhodamine dropwise at constant pressure under stirring. B hydrazide [synthesized in Example 1] was dissolved in 100 mL of ethanol solution, after the dropwise addition was completed, 1.3 mL of glacial acetic acid was added, stirred evenly, refluxed at 90°C for 22 hours, evaporated to dryness...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com