Amidino guanido substituted aromatic heterocyclic copmound and synthesis and use thereof
A technology of aromatic heterocycles and compounds, which can be used in medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc., and can solve the problems of high price of peptide inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Synthesis of amidino-substituted aromatic heterocyclic compounds
[0036] (1) Grind p-nitrobenzaldehyde, p-aminobenzoic acid and NAY zeolite evenly, under solvent-free conditions, cool to room temperature after microwave irradiation for 10 minutes, add methanol to dissolve, remove NAY zeolite by filtration, and concentrate the filtrate under reduced pressure to obtain (E)-4-(4-Nitrobenzimino)benzoic acid.
[0037] (2) Dissolve (E)-4-(4-nitrobenzimino)benzoic acid in anhydrous methanol, carefully add sodium borohydride at 0°C, react at room temperature for 2 hours, add water to the reaction solution, Adjust the pH to 6 with formic acid, extract with dichloromethane / methanol, and separate and purify with polyamide or silica gel to obtain 4-(4-nitrobenzylamino)benzoic acid.
[0038] (3) a. Under ice-bath conditions, feed dry HCl gas into methanol and make it saturated, add 6-cyano-2-naphthol, react overnight at room temperature, generate methyl 6-hydroxy-2-n...
Embodiment 2
[0042] Embodiment 2: BApNA (N-benzoyl-DL-arginine p-nitroaniline hydrochloride) assays the inhibitory activity of compound (III) to trypsin
[0043] experimental method:
[0044] Take a 96-well plate, and add 50 μl of compound (III) aqueous solution (0.061, 0.122, 0.245, 0.489, 0.977, 1.953, 3.906, 7.813 μg / ml) and 50 μl of human trypsin solution (0.1 mg / ml) into each well successively. ml) and 100 μl BApNA solution (prepared to a 0.5 mg / ml solution with pH 7.4, 0.05M Tris-HCl buffer), in the other 3 wells, replace compound (III) with 50 μl deionized water, and the addition of the remaining reagents Same as above, as a non-inhibition control, incubate the 96-well plate at 37°C for 10 min, then add 20 μl of glacial acetic acid to terminate the reaction, and immediately measure the absorbance at 405 nm using a microplate reader. Calculate the inhibitory rate of compound (III) to trypsin at different concentrations according to the absorbance value, and the calculation formula i...
Embodiment 3
[0053] Embodiment 3: the protective effect of compound (III) on mice with acute pancreatitis
[0054] experimental method:
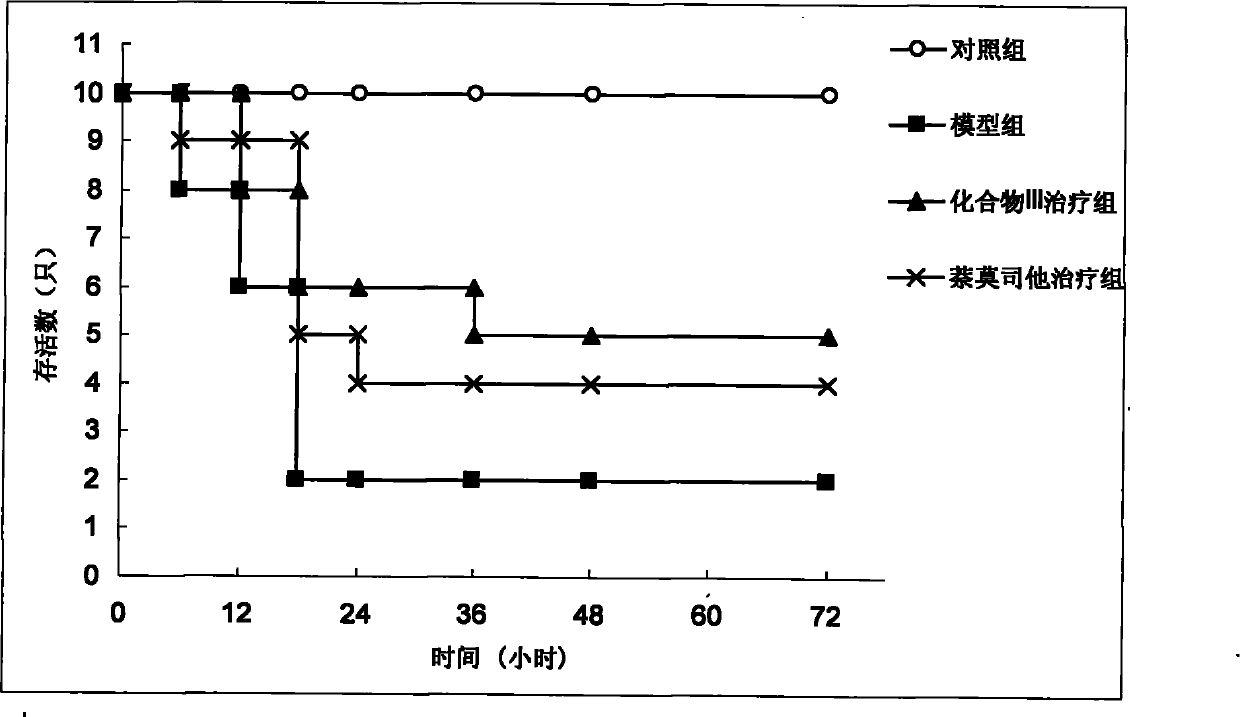
[0055] (1) Survival rate survey:
[0056] Get 40 KM mice and divide them into 4 groups randomly (n=10): control group, acute pancreatitis model group, acute pancreatitis model+compound (III) administration group, acute pancreatitis model+nafamostat The medicine group, wherein the preparation method of the model group is: 1 time per hour, 2 consecutive intraperitoneal injections of L-arginine (each dose is 4g / kg) to the mice to induce acute pancreatitis; the control group is only injected with normal saline; In pancreatitis model+compound (III) administration group, acute pancreatitis model+nafamostat administration group, compound (III) and nafamostat were injected intraperitoneally 1h after the last L-arginine injection. Medicine, the dose is 10mg / kg. The survival of each group was observed within 72 hours after administration.
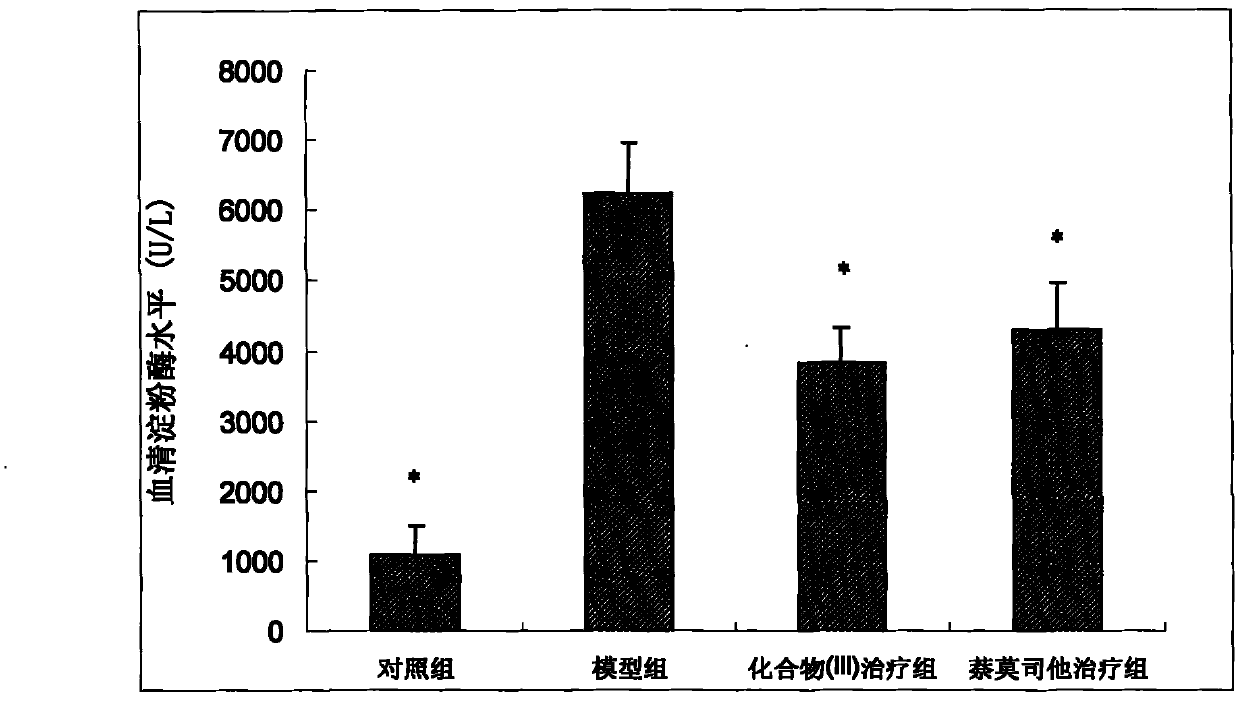
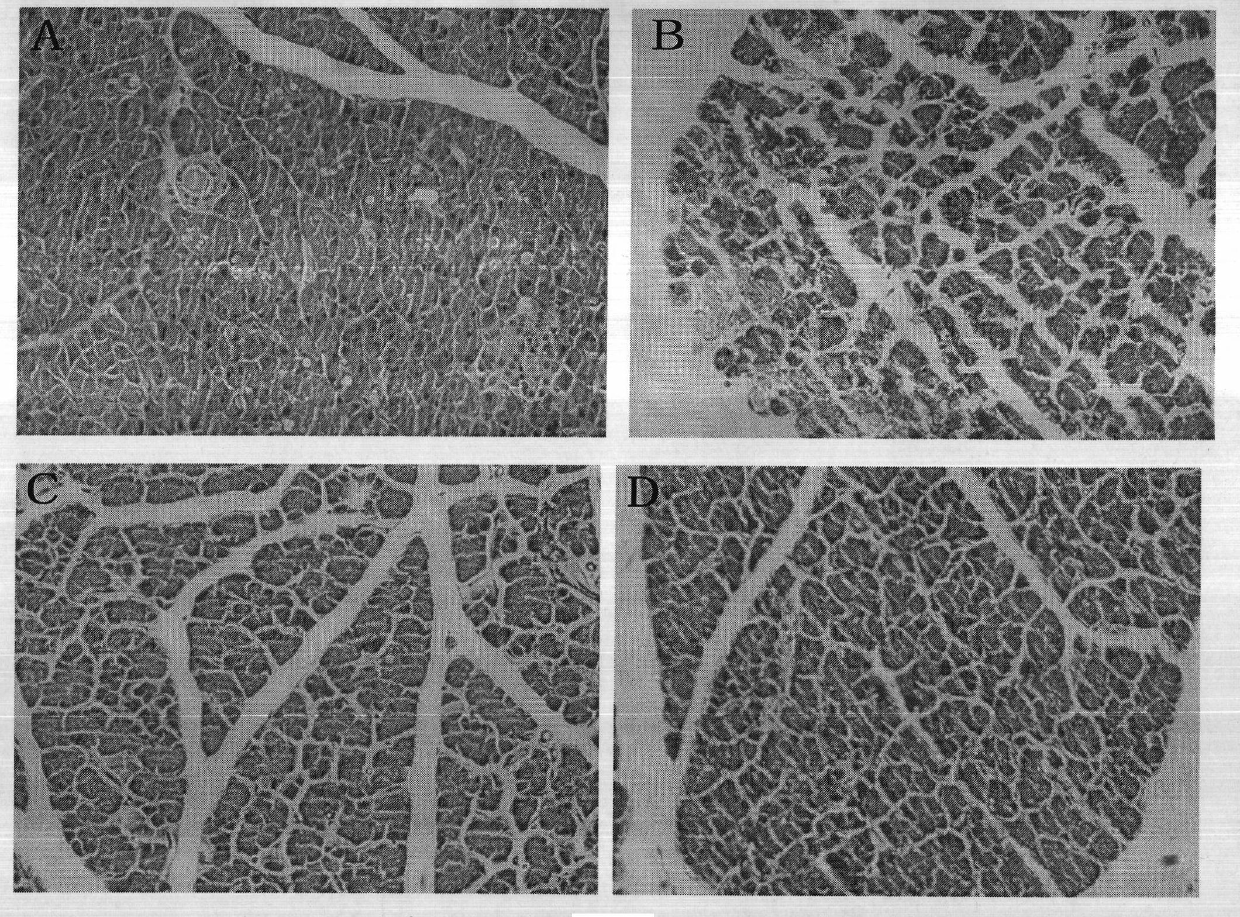
[0057] (2) Enzyme a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com