Organic substituted boric acid ester, boron affinity functional material using organic substituted boric acid ester as functional monomer as well as preparation and application of organic substituted boric acid ester
A borate and alcohol borate technology, which is applied in the field of new phenylboronate ligands and phenylboronic acid functionalized porous monolithic materials, can solve the problems of not reaching the scope of application and limiting applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Synthesis of 4-amino-2-(dimethylaminomethyl)phenylboronic acid pinacol ester (Formula 4)
[0038] The first step is the synthesis of 4-nitro-2-methylphenylboronic acid pinacol ester (formula 1)
[0039] 10.02 g 4-nitro-2-methyl bromobenzene, 13.00 g pinacol diboron, 12.80 g potassium acetate, [1,1'-bis(diphenylphosphine)ferrocene]palladium dichloride 0.42 The grams were put into a 500 ml three-necked flask, 220 ml dioxane was added, and the reaction was carried out at 100°C for 12 hours under the protection of nitrogen. After cooling to room temperature, 220 ml of ethyl acetate and 400 ml of water were added, and the organic layer was separated, washed with water twice, brine once, and dried with anhydrous magnesium sulfate. After filtration, it was concentrated to remove the solvent, and separated by column chromatography (EtOAc:Hexane=1:20, v / v) to obtain the compound represented by Formula 1, melting point: 46-47°C, yield 70%.
[0040] 1 H NMR (500 MHz, CDCl 3 ...
Embodiment 2
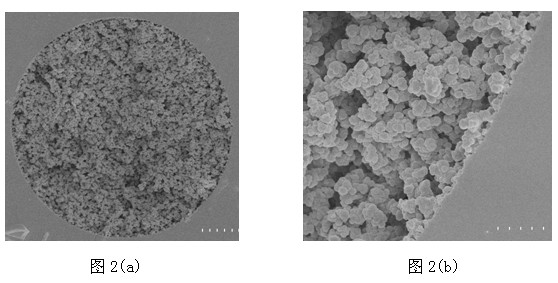
[0054] Example 2 Synthesis of monolithic materials
[0055] (1) Synthesis of capillary monolithic column: 240mg glycidyl methacrylate (GMA), 160mg polyethylene glycol diacrylate (PEGDA), 10mg azobisisobutyronitrile and 200mg dimethyl sulfoxide (DMSO) ,400mg 1,4-butanediol was mixed, vortexed and sonicated for 30 minutes, injected into the activated capillary, sealed both ends of the capillary with rubber pads, and then placed in a water bath for 18 hours at 75°C. After finishing, flush out the porogen (DMSO, dodecanol) to obtain a capillary monolithic column.
[0056] (2) Synthesis of a monolithic column of conventional size: 2.4g glycidyl methacrylate (GMA), 1.6g polyethylene glycol and acrylate (PEGDA), 50mg azobisisobutyronitrile and 2.0g dimethyl methacrylate Sulfone (DMSO), 4.0g 1,4-butanediol mixed, vortex and ultrasonic for 30 minutes, add the prepared mixed solution to a conventional column, seal both ends and put it in a water bath at 75°C After reacting for 24h, the con...
Embodiment 3
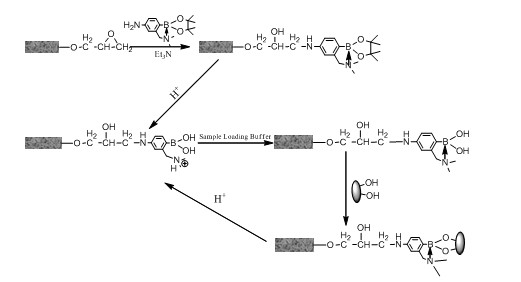
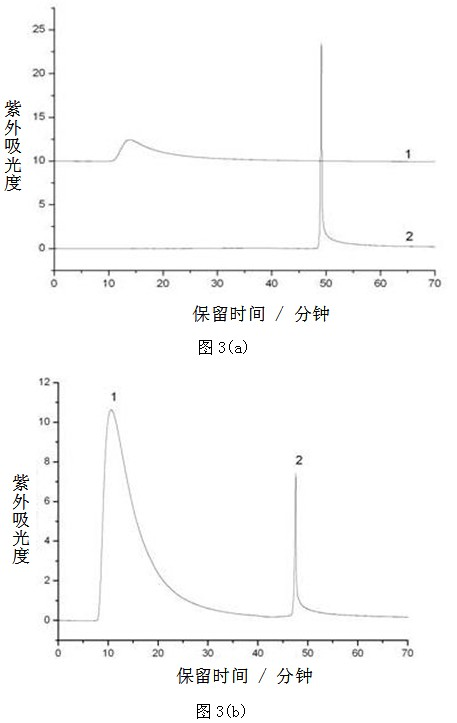
[0058] Example 3 Modification of synthetic functionalized phenylborate on the surface of monolithic column
[0059] (1) Surface modification of monolithic column materials (including capillary columns and conventional columns): take a certain amount of 3-dimethylaminomethylaniline-4-pinacol borate, dissolve it in acetonitrile, and then add a certain amount of three Ethylamine. The prepared solution was injected into the monolithic column and reacted at 60°C for 24h. After the reaction, the unreacted reagents in the monolithic column were washed out with pure acetonitrile to obtain the modified monolithic material.
[0060] (2) Surface modification of massive monoliths: Take a certain amount of 3-dimethylaminomethylaniline-4-pinacol borate, dissolve it in a certain volume of acetonitrile, and then add a certain amount of triethylamine, Then soak the block-shaped monolithic material in the above-prepared solution, and then heat it at 60°C for 24h, and then use the Soxhlet extraction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com