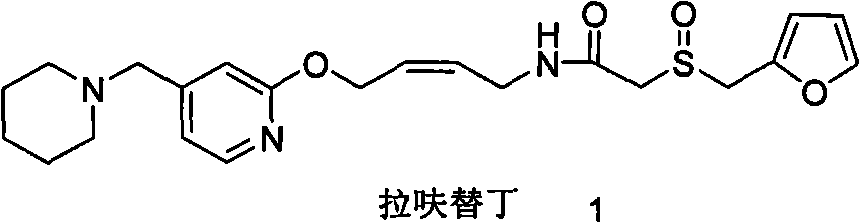
Method for preparing lafutidine by virtue of aminolysis
A technology of lafutidine and methylamine, applied in the new field of chemical synthesis, can solve the problems of high synthetic cost of lafutidine, reduce the total yield of synthetic lafutidine, etc., and achieve simplified post-treatment process, The effect of avoiding loss and improving synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0058] Experimental example: the effect of hydrazine on the production of dihydrolafutidine
[0059] In order to verify the effect of hydrazine on the production of dihydrolafutidine, the inventors designed and carried out the following experiments.
[0060] Dissolve 1 equivalent of the compound of formula 3 obtained by hydrazinolysis in a solvent, add 10 equivalents of hydrazine hydrate, heat and reflux for a certain period of time, evaporate the solvent to dryness, dissolve with ethyl acetate, wash with saturated sodium chloride solution, and dry over anhydrous magnesium sulfate. The compound of formula 3 obtained by evaporating ethyl acetate, further condenses with 2-furan methylsulfinyl acetate p-nitrophenol ester (compound of formula 4) to obtain lafutidine, check wherein dihydrolafutidine with liquid chromatography Ding impurity content.
[0061]
[0062] In the above test, the compound of formula 3 and the compound of formula 4 were condensed to obtain lafut...
Embodiment 2
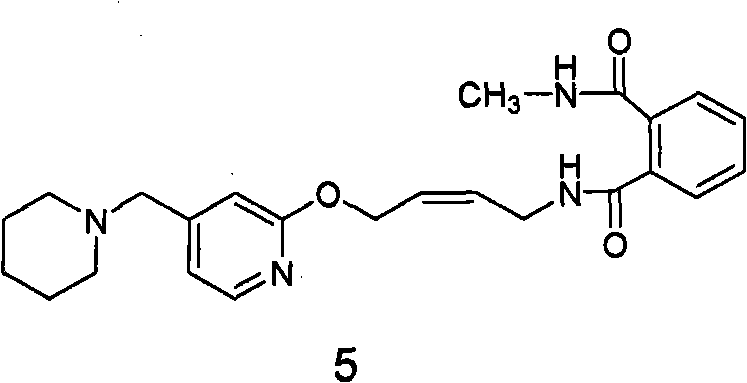
[0071] Example 2: cis-N-methyl-N-(4-(4-piperidinylmethyl)pyridinyl-2-oxo)-2- Enyl) phthalamide (formula 5 compound) preparation
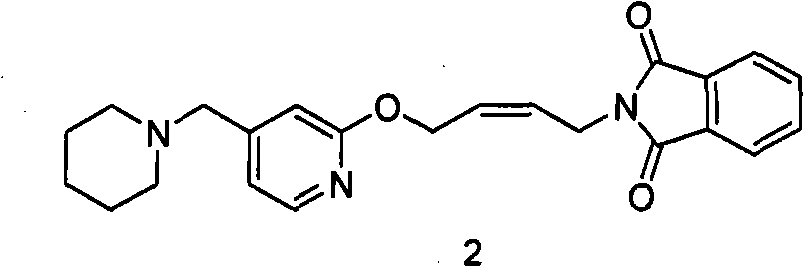
[0072] 200 grams (0.394 moles) of N-(4-(4-piperidinylmethyl) pyridyl-2-oxo)-2-ene butyl) phthalimide (formula 2 compound) Forate was suspended in 2L of absolute ethanol, and 540 ml (3.94 mol) of 25% methylamine aqueous solution was added under stirring at room temperature. The reaction solution was quickly clarified, then cloudy, and gradually produced a large amount of white solid. Filtration obtains this solid, proves that this solid is cis-N-methyl-N-(4-(4-piperidylmethyl)pyridyl-2-oxo)-2- Butyl) phthalamide (compound of formula 5). 1 H NMR (CDCl 3 ): δ1.41-1.42 (2H, m, 3-CH 2 ); 1.52-1.57 (4H, m, 2-CH 2 ); 2.33 (4H, s, 1-CH 2 ); 2.89-2.90 (3H, d, 4-CH 3 ); 3.36 (2H, m, 5-CH 2 ); 4.12-4.14 (2H, t, 7-CH 2 ); 4.93-4.94 (2H, d, 6-CH 2 ); 5.67-5.87 (2H, m, 8-H, 9-H); 6.89-7.75 (7H, m, Ar); MS: 423 (M+1).
[0073]
Embodiment 3
[0074] Embodiment 3: Lafutidine is prepared by aminolysis method of the present invention
[0075] 1. The preparation of cis-4-(4-piperidinylmethyl)pyridyl-2-oxo)-2-butenamine (formula 3 compound)
[0076] 200 grams (0.394 moles) of N-(4-(4-piperidinylmethyl) pyridyl-2-oxo)-2-ene butyl) phthalimide (formula 2 compound) Forate was suspended in 2L of absolute ethanol, and 540 ml (3.94 moles) of 25% methylamine aqueous solution was added under stirring at room temperature. The reaction solution was quickly clarified, then cloudy, and gradually produced a large amount of white solid (compound of formula 5). After continuing to stir at room temperature for 8 hours, the reaction solution became clear, and 500 milliliters of 20% NaOH solution (95 grams were dissolved in 500 milliliters of water) was added for reaction for 2 hours. TLC showed that N-(4-(4-piperidinylmethyl)pyridine Base-2-oxo)-2-enbutyl)phthalimide (compound of formula 2) has been completely converted to cis-4-(4-p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com