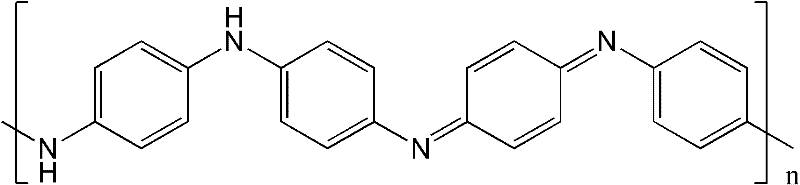
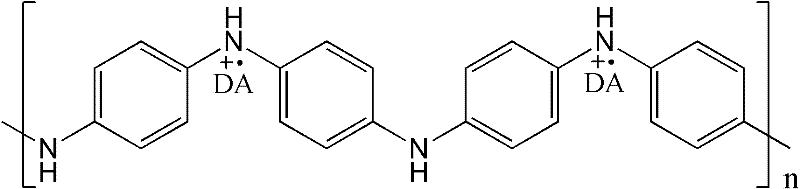
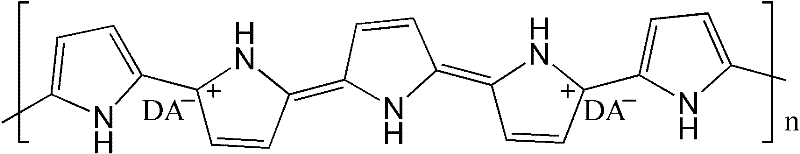
Electric conduction polymer and synthesis method thereof and electroactive electrode with surface covered with electric conduction polymer
A technology of conductive polymers and synthesis methods, applied in chemical instruments and methods, synthetic resin layered products, layered products, etc., can solve the problem of reducing the conductivity of conductive polymers, reducing the performance of electrodes and sensors, reducing the conductivity of materials and Electrochemical activity and other issues, to achieve the effect of a simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Phytic acid (functionality 6, containing 6 phosphate radicals) doped polyaniline hydrogel
[0062] First configure 20 ml of ammonium persulfate oxidant aqueous solution with a concentration of 2M, and configure 25 ml of monomer aqueous solution mixed with aniline and phytic acid. The ratio of the amount of substances is ammonium persulfate: aniline: phytic acid=3:6:1. The two solutions are then mixed, and within minutes, polymerization occurs to produce a polyaniline hydrogel. It was observed that the color of the solution changed to dark green and the solution lost fluidity. Finally, dialysis was performed in deionized water to purify the hydrogel to remove impurities such as ions and oligomers to obtain a hydrogel with a water content of 72%. In this reaction, the mixing ratio of the reagents can be changed within a certain range (for example: the ratio of the amount of phosphoric acid groups contained in aniline and phytic acid can form a gel in the ra...
Embodiment 2
[0063] Embodiment 2: Phytic acid doped polyaniline hydrogel (water content 34%)
[0064] First prepare 1ml of ammonium persulfate oxidizing agent aqueous solution containing 0.286g, and configure the monomer aqueous solution mixed with aniline (0.458ml) and phytic acid (0.921ml) (the concentration can be deduced from the water content of the hydrogel). The two solutions are then mixed, and within minutes, polymerization occurs to produce a polyaniline hydrogel. It was observed that the color of the solution changed to dark green and the solution lost fluidity. A hydrogel with a water content of 34% was obtained. The ionic conductivity of the resulting hydrogel was 0.030 S cm -1 .
Embodiment 3
[0065] Embodiment 3: Phytic acid doped polyaniline hydrogel (water content 85%)
[0066] First configure 2.5 ml of ammonium persulfate oxidant aqueous solution containing 0.286 g, and configure 6.5 ml of monomer aqueous solution mixed with aniline (0.458 ml) and phytic acid (0.921 ml). The two solutions are then mixed, and within minutes, polymerization occurs to produce a polyaniline hydrogel. It was observed that the color of the solution changed to dark green and the solution lost fluidity. A hydrogel with a water content of 85% was obtained. The ionic conductivity of the resulting hydrogel was 0.017 S cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com