NmpC subunit vaccine of salmonella paratyphi A and preparation method thereof
A Salmonella and Paratyphoid technology, applied in the field of Salmonella Paratyphi A subunit vaccine and its preparation, can solve the problems of no preparation of Salmonella Paratyphi A vaccine, few types of NmpC, etc., and achieve easy cultivation and fermentation, and low production cost , the effect of simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
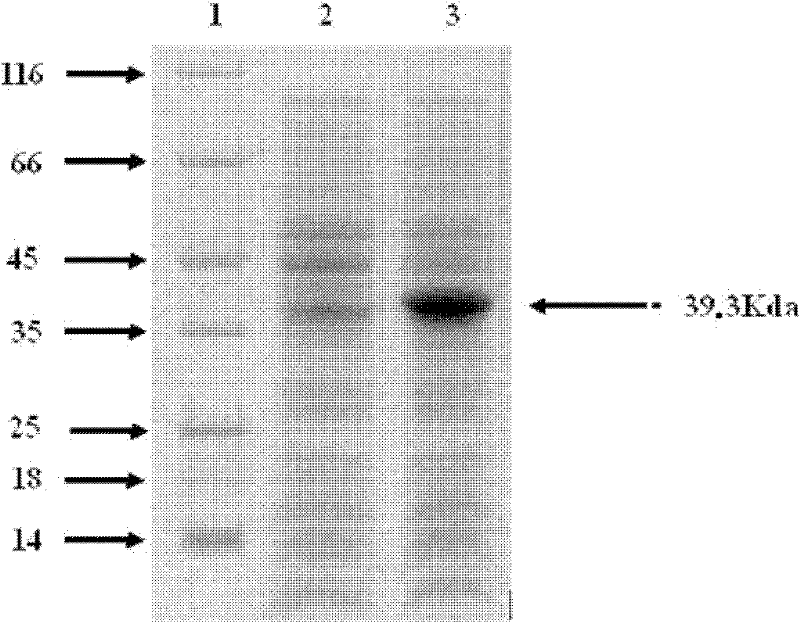
[0036] Embodiment 1: the preparation of Salmonella paratyphi A membrane protein NmpC
[0037] 1. Acquisition of the membrane protein NmpC gene of Salmonella paratyphi A
[0038] Using the genome of Salmonella paratyphi A 50973 strain (source: China Medical Bacteria Collection Management Center) as a template, entrust a DNA synthesis company to synthesize upstream and downstream primers
[0039]NmpC-f: 5'-GGGAATTCCATATGGCCGAGGTATATAACAAAGACG-3';
[0040] NmpC-r: 5'-CCGCTCGAGTTAGTGGTGGTGGTGGTGGTGGAACTGGTAGTTCAGACCA-3'.
[0041] Among them, NmpC-f and NmpC-r are used to amplify the NmpC protein gene, and introduce NdeI and XhoI restriction sites and 6×His tags. PCR amplification was performed using Taq plus enzyme: pre-denaturation at 94°C for 10 min; 30 cycles (94°C for 30 s, 52°C for 30 s, 72°C for 1 min); 72°C for 7 min. Amplified products were identified by 1% agarose gel electrophoresis.
[0042] 2. Construction of recombinant expression vector NmpC-pET30a
[0043] Afte...
Embodiment 2
[0052] Embodiment 2: Animal immunization experiment of NmpC protein
[0053] 1. Animal immunity
[0054] BALB / c mice were divided into 3 groups, 20 in each group, 10 of which were blood collected, and 10 were challenged. Immunization was carried out at 0, 2, and 4 weeks by intramuscular injection. Group A is healthy mice; Group B uses aluminum adjuvant; Group C uses NmpC protein + aluminum adjuvant. The immunization dose is 0.2ml per mouse, containing 10μg of antigen and 0.2mg of aluminum adjuvant. Serum collection was performed 10 days after each immunization. The first and second time blood was collected from the canthus venous plexus, and the third time blood was collected from the eyeball. Serum total IgG antibody was measured by ELISA method, and the inter-group differences of each group of data were analyzed by SPSS software. The results showed that the titer of the mouse IgG antibody against NmpC protein was very high.
[0055] 2. Antivirus protection experiment ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com