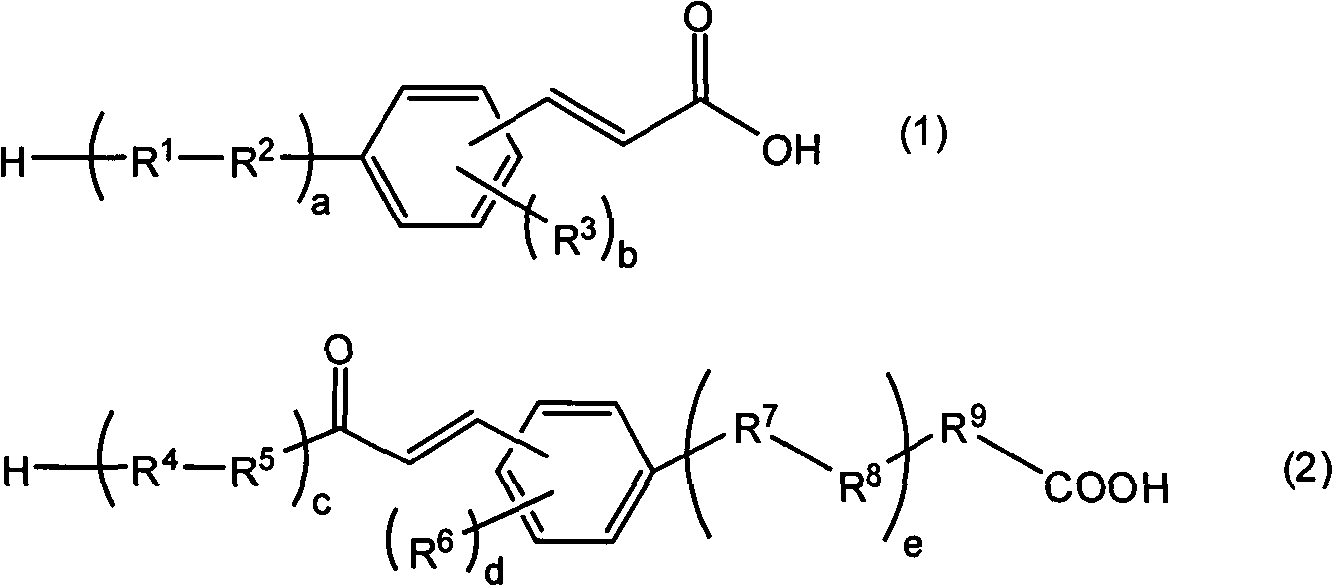
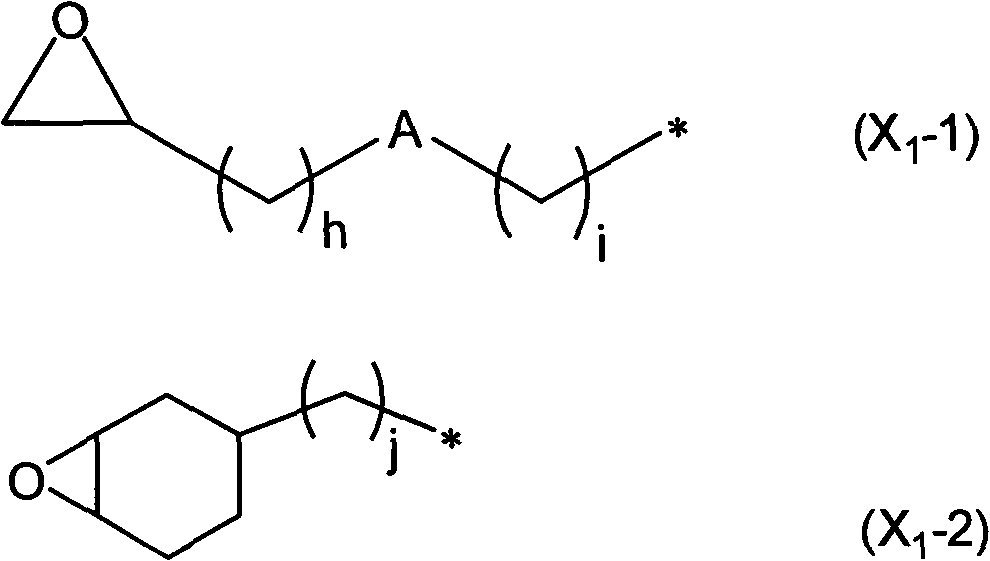
Composition for organic EL luminophor orientation controlling, organic EL luminophor orientation controlling film, and organic EL element and fabricating method thereof
A technology of orientation control and manufacturing method, which is applied in semiconductor/solid-state device manufacturing, optical components, electroluminescent light sources, etc., can solve the problems of reduced luminous efficiency and difficult light-emitting layer, achieve good photo-orientation sensitivity, and realize luminescence Efficiency stability, the effect of excellent polarized luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0406] In the reaction vessel equipped with stirrer, thermometer, dropping funnel and reflux condenser, add: 100.0g 2-(3,4-epoxycyclohexyl) ethyltrimethoxysilane (ECETS), 500g methylisobutyl Ketone and 10.0 g of triethylamine were mixed at room temperature.
[0407] Next, after dropping 100 g of deionized water from the dropping funnel over 30 minutes, the mixture was continuously mixed under reflux, and reacted at 80° C. for 6 hours. After the reaction, the organic layer was taken out, washed with a 0.2% by mass ammonium nitrate aqueous solution until the water became neutral, and the solvent and water were distilled off under reduced pressure to obtain a polyorganosiloxane having an epoxy group. Viscous transparent liquid.
[0408] The polyorganosiloxane with epoxy group is carried out 1 After the H-NMR analysis, a peak based on the epoxy group was obtained by theoretical intensity in the vicinity of chemical shift (δ)=3.2 ppm, and it was confirmed that no side reaction of...
Synthetic example 2
[0412] 20 g of 4-bromodiphenyl ether, 0.18 g of palladium acetate, 0.98 g of tris(2-tolyl)phosphine, 32.4 g of triethylamine, and 135 mL of dimethylacetamide were mixed in a 500 mL three-necked flask equipped with a condenser. Next, 7 g of acrylic acid was added to the mixed solution with a syringe, and stirred. This mixed solution was further heated and stirred at 120° C. for 3 hours. After confirming the completion of the reaction by TLC, the reaction solution was cooled to room temperature. After filtering off the precipitate, the filtrate was poured into 300 mL of 1N hydrochloric acid aqueous solution, and the precipitate was recovered. This precipitate was recrystallized under a solution of ethyl acetate and hexane at a ratio of 1:1, thereby obtaining 8.4 g of a compound represented by the following formula (K-1) (specific cinnamic acid derivative (K-1)) .
[0413]
Synthetic example 3
[0415] In a 300 mL three-necked flask equipped with a condenser, 6.5 g of 4-fluorophenylboronic acid, 10 g of 4-bromocinnamic acid, 2.7 g of tetrakis(triphenylphosphine)palladium, 4 g of sodium carbonate, 80 mL of tetrahydrofuran, and 39 mL of pure water were mixed. Continue heating and stirring the reaction solution at 80° C. for 8 hours, and confirm with TLC after the reaction is completed. After the reaction solution was cooled to room temperature, it was poured into 200 mL of 1N hydrochloric acid aqueous solution, and a solid was precipitated by filtration. The obtained solid was dissolved in ethyl acetate, and the liquid separation was washed with 100 mL of 1N aqueous hydrochloric acid solution, 100 mL of pure water, and 100 mL of saturated brine in this order. Next, the organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated off. The obtained solid was vacuum-dried to obtain 9 g of a compound represented by the following formula (K-2) (s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com