Water-soluble derivatives of edaravone, preparation method and application thereof
A technology of derivatives and synthesis methods, applied to water-soluble derivatives of edaravone and the fields of preparation and application thereof, can solve the problem of harsh process conditions, low oral bioavailability of edaravone, and water solubility of edaravone. It can improve the problems such as insignificant improvement, and achieve the effect of improving water solubility and improving drug bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
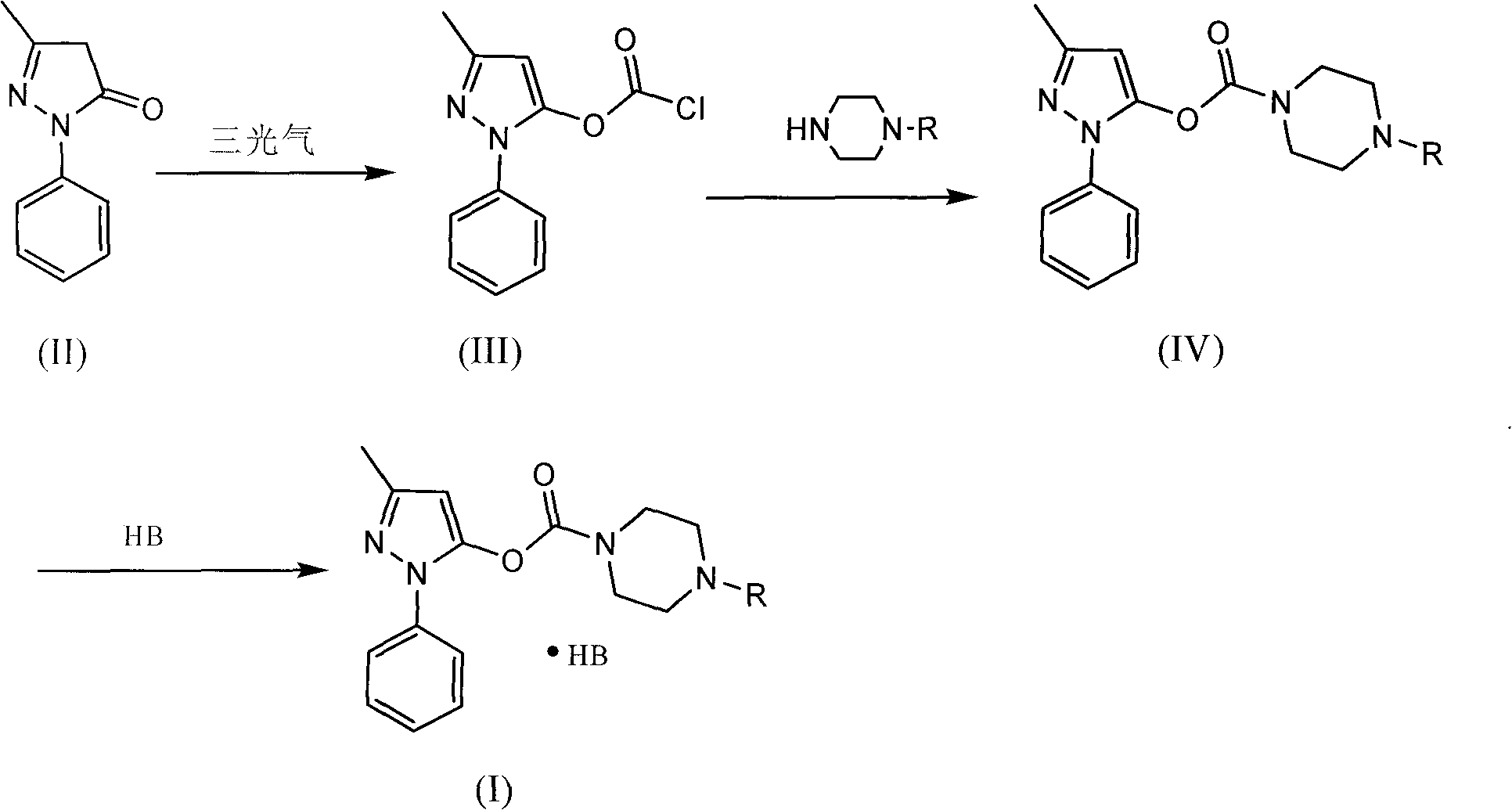
[0034] Preparation of 1-piperazineformyl edaravone ester (compound 1)
[0035] in dry N 2 Flow down, add 15ml of dry dichloromethane, 2.0g of edaravone, 0.93g of anhydrous pyridine into a 50ml dry three-necked flask, and stir until completely dissolved. Dissolve 1.14g of triphosgene in 5ml of dry dichloromethane, add it dropwise slowly, and react at -10°C for 2-3 hours. After the reaction was completed, the pyridinium salt was filtered off, and the filtrate was placed in a constant pressure dropping funnel for subsequent use.
[0036] in dry N 2Flowing down, add 5ml of dry dichloromethane and 5.1g of cbz-piperazine into a 50ml dry three-necked flask, and stir until completely dissolved. Then the above filtrate was slowly dropped into it, and reacted at -5°C for 0.5 hours. After the reaction was completed, it was washed with acidic water at pH=1 until no cbz-piperazine was present in the organic phase. The liquid was separated, and then the dichloromethane was vacuum-dried ...
Embodiment 2
[0039] Preparation of 4-methyl-1-piperazinecarboyl edaravone ester (compound 2)
[0040] in dry N 2 Flowing down, add 20ml of dry chloroform, 2.0g of edaravone, and 1.5g of anhydrous pyridine into a 50ml dry three-necked flask, and stir until completely dissolved. Dissolve 1.5g of triphosgene in 5ml of dry chloroform, add it dropwise slowly, and react at -15°C for 2-3 hours. After the reaction was completed, the pyridinium salt was filtered off, and the filtrate was placed in a constant pressure dropping funnel for subsequent use.
[0041] in dry N 2 Add 10ml of dry chloroform and 3.0g of methylpiperazine to a 50ml dry three-necked flask, stir in an ice bath; then slowly drop the above filtrate into it, and react at 0°C for 0.5 hours. After the reaction, wash with water until there is no methylpiperazine in the organic phase, separate the layers, and evaporate chloroform to obtain an oily substance.
[0042] The above oil was dissolved in 20ml of ethyl acetate, and a satur...
Embodiment 3
[0044] Preparation of 4-methyl-1-piperazinecarboyl edaravone ester (compound 2)
[0045] in dry N 2 Add 20ml of dry dichloromethane, 2.0g of methylpiperazine, and 2.52g of sodium bicarbonate into a 50ml dry three-necked flask, and stir. Dissolve 1.97g of triphosgene in 10ml of dry dichloromethane, add it dropwise slowly, and react at -10°C for 1 hour. After filtration, the filtrate was evaporated to dryness under reduced pressure to obtain a light yellow solid.
[0046] in dry N 2 Add 30ml of dry dichloromethane, 3.5g of edaravone, and 1.9g of pyridine into a 100ml dry three-necked flask, and stir in an ice bath. Then 10 ml of the above solid dissolved in dichloromethane was slowly dropped into it, and reacted at 20° C. for 5 hours. After the reaction, wash with water until there is no pyridine in the organic phase, separate the layers, and evaporate dichloromethane to obtain an oily substance.
[0047] The above oil was dissolved in 30ml of acetone, and a saturated HCl s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com