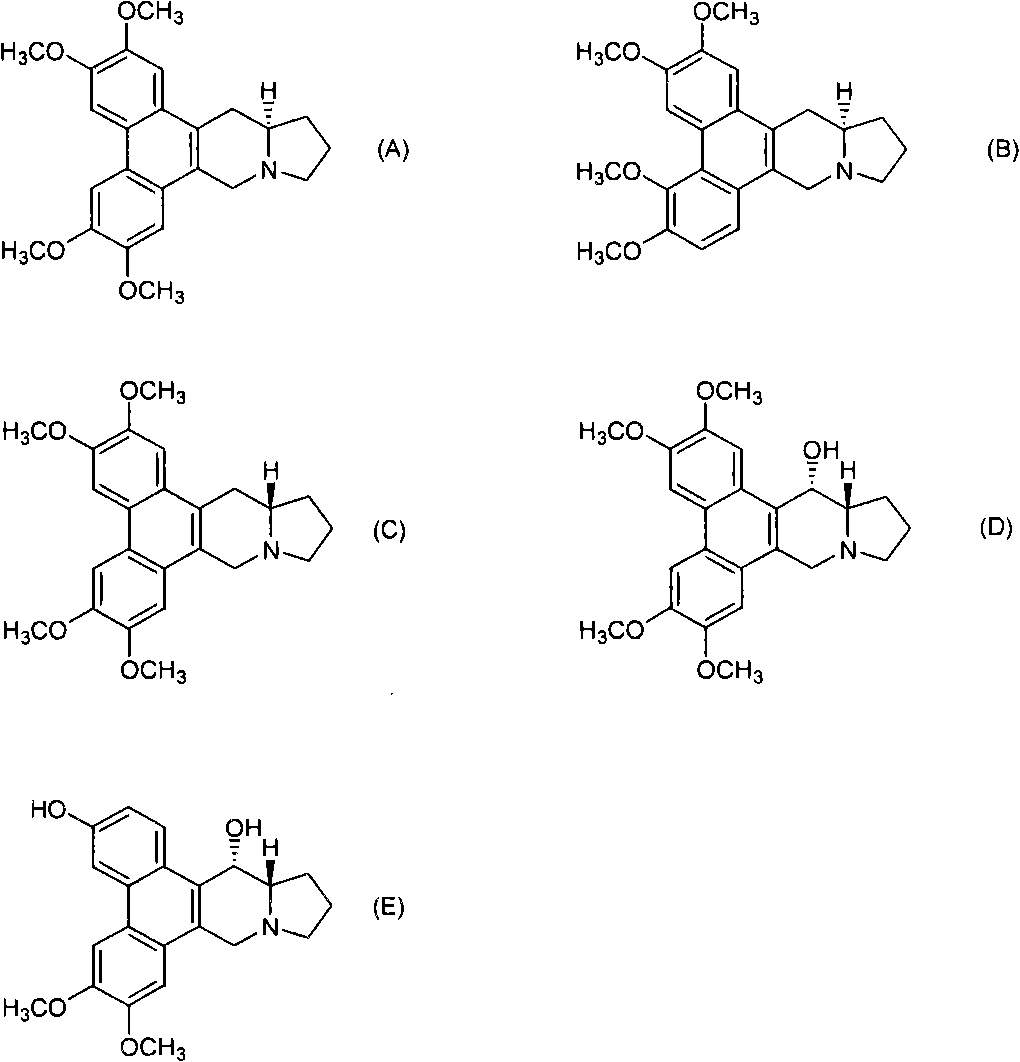
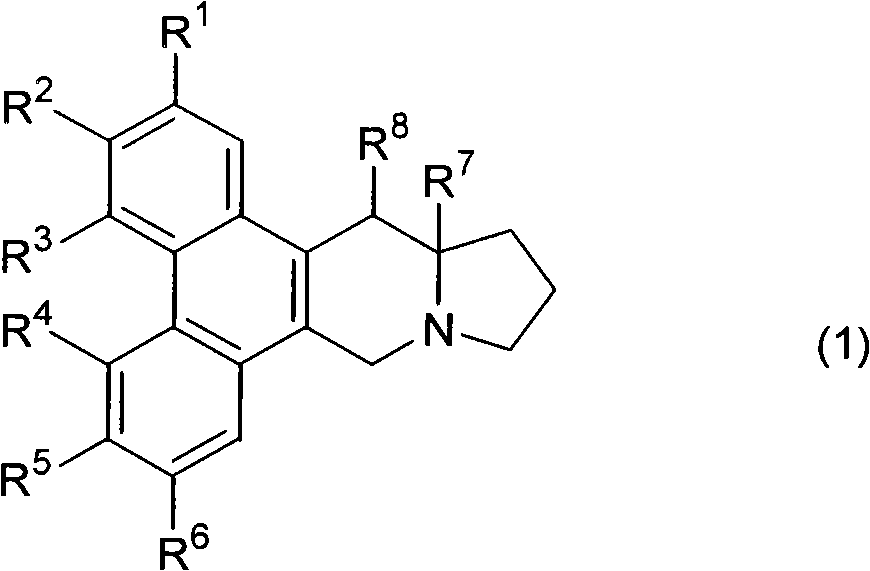
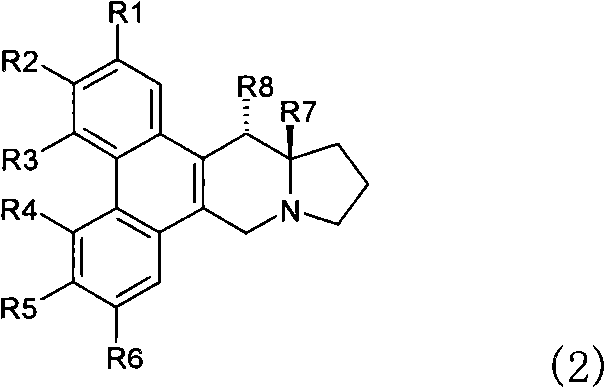
Phenanthrene-indorisidine derivatives and nfκb inhibitors using them as active ingredients
A technology of active ingredients and substances, applied in the field of novel phenanthrene indolizidine alkaloid compounds or their salts, can solve the problems of strong cytotoxicity and difference, and achieve excellent solubility, less side effects, and excellent NFκB inhibitory effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0194] Hereinafter, examples are shown and the present invention will be described in more detail, but the present invention is not limited thereto.
[0195] The phenanthrene indolizidine alkaloid of the present invention is synthesized according to the reaction pathway of the following steps 1-10. When the substituent represented by R needs to be protected, an appropriate protecting group is used for the reaction.
[0196]
Synthetic example 1
[0198] Synthetic R 1 ~R 6 Compounds represented by the following groups. The operation of steps 1 to 10 is as follows.
[0199] [Table 1]
[0200] Compound 1
[0201] R 1 R 2 R 3 R 4 R 5 R 6 H H H H OCH 3 OCH 3
[0202] Step 1: Synthesis of stilbene
[0203] In an eggplant-shaped flask, in 3,4-dimethoxyphenylacetonitrile 10.0g (56.43mmol) and benzaldehyde 5.99g (56.43mmol, 1.0eq.) in ethanol 150mL suspension, in an argon atmosphere, 380 mg (5.64 mmol, 0.1 eq.) of sodium ethoxide was added with stirring at room temperature, and heated to reflux (oil bath temperature: 85° C.). After 3 hours, the disappearance of the raw material was confirmed, and the reaction solution was cooled with an ice bath. The precipitated solid was suction-filtered with a Buchner funnel and a suction filter flask, and washed with 100 mL of methanol (twice). Drying was carried out at 60° C. under reduced pressure to obtain 13.70 g (92%) of...
Synthetic example 2
[0247] Synthetic R 1 ~R 6 Compounds represented by the following groups. The operations and the yield of each operation are shown below.
[0248] [Table 2]
[0249] Compound 29
[0250] R 1 R 2 R 3 R 4 R 5 R 6 Oh H H H OCH 3 OCH 3
[0251] Process 1
[0252] Yield: quant
[0253] 1 HNMR (400MHz, CDCl 3 )δ: 3.93 (3H, s), 3.96 (3H, s), 5.14 (2H, s), 6.92 (1H, dd, J=8.5Hz), 7.01-7.07 (1H, m), 7.14 (1H, d , J=2.4Hz), 7.26 (1H, dd, J=2.4, 8.5Hz), 7.31-7.48 (8H, m), 7.53-7.56 (1H, m)
[0254] Process 2
[0255] Yield: 88.7%
[0256] Separated as a mixture of positional isomers (isomer ratio 66:34) with respect to the benzyloxy group on the aromatic ring
[0257] Process 3
[0258] Yield: 97.6%
[0259] Separated as a mixture of positional isomers (isomer ratio 66:34) with respect to the benzyloxy group on the aromatic ring
[0260] Process 4
[0261] Yield: quant
[0262] Separated as a mixture of positional is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com