Short peptide having kidney protection function as well as preparation method and application thereof
A kidney protection and effect technology is applied to short peptides with kidney protection effect and the fields of preparation and application thereof, which can solve the problems of promoting tumor formation, increasing adverse reactions, and promoting thrombosis, and achieves easy operation, high preparation efficiency, biological Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
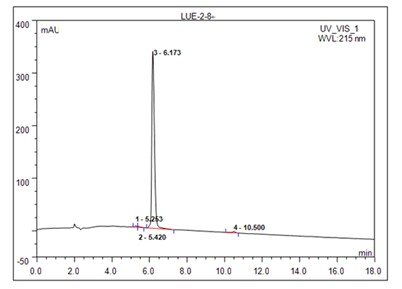
[0030] Example 1: Preparation of Helix B surface peptide (HBSP):
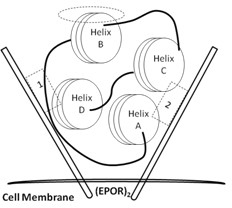
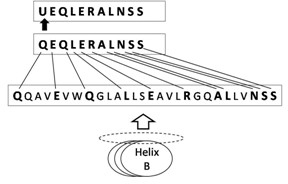
[0031] HBSP is a short peptide with renal protection. Its sequence and structure are derived from erythropoietin (EPO) B-helix surface peptide (Helix B surface peptide, HBSP). The specific amino acid sequence is QEQLERALNSS. Such as figure 1 Shown, for EPO and (EPOR) 2 Binding spatial conformation diagram, the four subunit helices of EPO (Helix A-D) form a compressed spherical structure through hydrophobic interactions, and 1 and 2 marked by dotted lines (square dotted line boxes) are the binding parts of EPO and high-affinity receptors , while the hydrophilic Helix B is located on top of the EPO spheroids (circular dashed box). Such as figure 2 Shown is a schematic diagram of the HBSP sequence, and 11 amino acid residues are extracted from the hydrophilic surface of the Helix B helix to form HBSP. Glutamate at the N-terminus can spontaneously cyclize to pyroglutamate, forming pHBSP (peptide chain shown at...
Embodiment 2
[0050] Example 2: Effect of HBSP on renal function after renal ischemia-reperfusion injury (ischemia reperfusion, IR) in rats:
[0051] a. Establish a model of renal ischemia-reperfusion injury in rats: inject ketamine solution (purchased from the Experimental Animal Center of Shanghai Zhongshan Hospital, specifications: 2ml: 0.1g) intraperitoneally at a dose of 0.12g / kg, and fix it on a plate after anesthesia. The abdominal wall was incised at the midline to enter the abdominal cavity, and the right kidney and renal pedicle were carefully separated to expose the renal pedicle. The renal pedicle was clamped with non-injured vascular clips, and the left kidney and renal pedicle were treated in the same way. After the renal pedicle vessel was blocked for 45 minutes, the color of the kidney turned purple and black, indicating that the clipping was successful. After the vascular clamp was released, the kidney showed mottled changes, indicating that the blood perfusion was restored....
Embodiment 3
[0056] Example 3: Effect of HBSP on renal tubular necrosis caused by renal ischemia-reperfusion injury in rats:
[0057] a. Paraffin-embedded the rat kidney tissue specimens obtained in Experiment 1, made sections, and stained with conventional HE methods.
[0058] b. Observe the pathological changes of kidney tissue in each group under the light microscope, and grade the renal tubular injury pathologically (0=normal kidney; 1=minimal damage, involving 75% of the cortex and outer medulla).
[0059] Such as Figure 6 Shown is the HE staining and damage pathological score (200x) map of kidney tissue section (compared with Vehicle group, *P ), the experimental results showed that the rats in the IR group showed obvious pathological changes in the renal tubules, such as blurred boundaries, swelling, vacuolar degeneration and necrosis, etc., while the pathological changes in the renal tubules of the rats in the HBSP treatment group were significantly alleviated, and the renal tubu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com