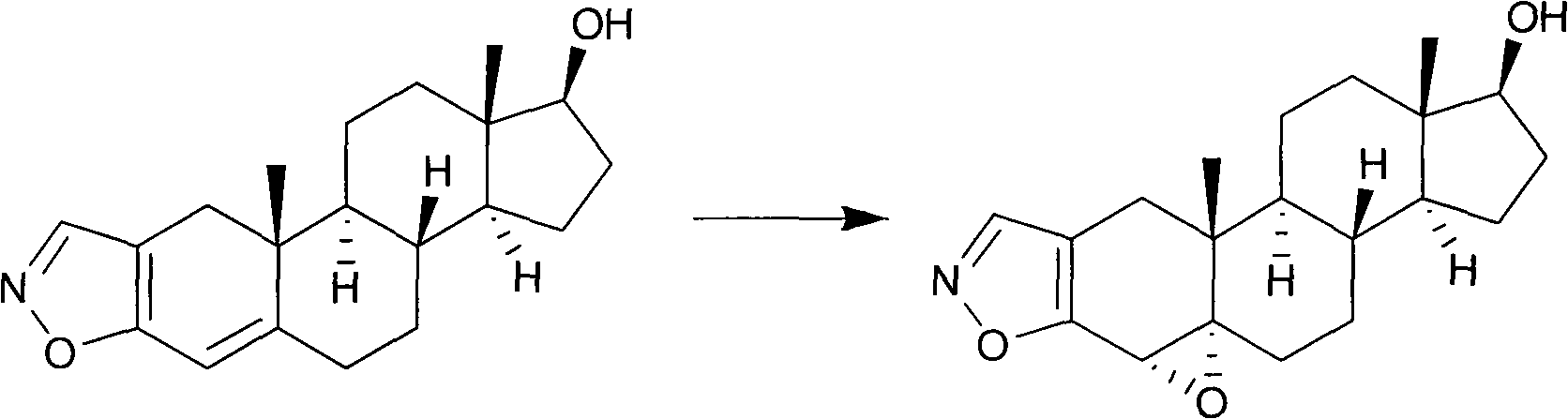
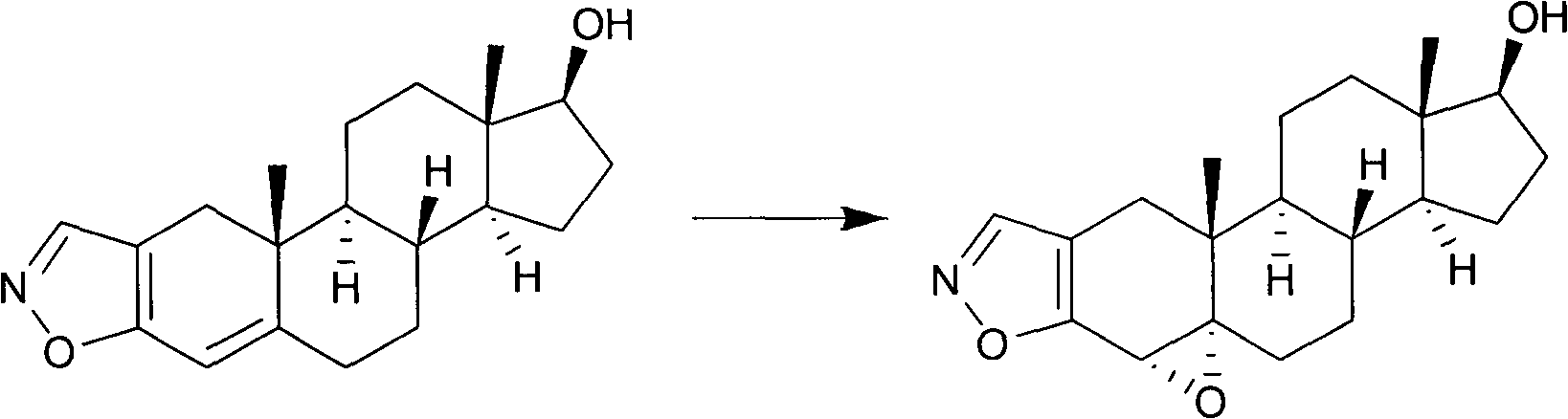
Preparation method of moguisteine intermediate 17 beta-hydroxyl-4 alpha, 5 alpha-epoxy androstane (2,3-d) isoxazole
A technology of epoxyandrostane and 3-d, which is applied in the field of preparation of Mojostein intermediate 17β-hydroxy-4α,5α-epoxyandrostano(2,3-d)isoxazole, can Solve problems such as the reduction of epoxide selectivity, and achieve the effect of high selectivity, high yield and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 17β-hydroxyl-4α, 5α-epoxyandrostano[2,3-d]isoxazole
[0021] Add 3.13g (0.01mol) of 17β-hydroxy-4-androstano[2,3-d]isoxazole into a 100ml three-necked flask, then add 50ml of benzene, stir magnetically, and control the temperature at 0°C. Under temperature, add 22.6mg (0.1mmol) diazepam Schiff base (E)-4--(pyridine-2-amino) benzoic acid, 25mg (0.1mmol) rhenium trioxide and 2.27g (0.02mol) 30%H 2 o 2 Aqueous solution, after stirring for 6 hours, stop the reaction, add a little sodium thiosulfate to remove excess H 2 o 2 , the catalyst was removed by filtration, and the residue was recrystallized with acetone to obtain 2.75 g of 17β-hydroxy-4α,5α-epoxyandrostano[2,3-d]isoxazole after evaporating the solvent, with a yield of 85.0%.
[0022] Melting point 204~206℃;
[0023] 13 C NMR (CDCl 3 )6: 11.1 (s, _C-18), 16.8 (s, C-19), 20.8 (s, C-11), 23.5 (s, C-15), 27.9 (s, C-7), 30.0 ( s, C-6), 30.2 (s, C-16), 30.4 (s, C-10), 35.9 (s, C-1), 36.5 ...
Embodiment 2
[0024] Example 2: Preparation of 17β-hydroxyl-4α, 5α-epoxyandrostano[2,3-d]isoxazole
[0025] Add 3.13g (0.01mol) of 17β-hydroxy-4-androstano[2,3-d]isoxazole into a 100ml three-neck flask, then add 50ml of dichloromethane, stir magnetically, and control the temperature at 10°C. At this temperature, add 22.6mg (0.1mmol) diazepam Schiff base (E)-4--(pyridine-2-amino)benzoic acid, 25mg (0.1mmol) methyl rhenium trioxide and 2.27g (0.02 mol)30%H 2 o 2 Aqueous solution, after stirring for 5 hours, stop the reaction, add a little sodium thiosulfate to remove excess H 2 o 2 , the catalyst was removed by filtration, and the residue was recrystallized with acetone to obtain 2.03 g of 17β-hydroxyl-4α,5α-epoxyandrostano[2,3-d]isoxazole after evaporating the solvent, with a yield of 62.8%.
Embodiment 3
[0026] Example 3: Example 2: Preparation of 17β-hydroxyl-4α, 5α-epoxyandrostano[2,3-d]isoxazole
[0027] Add 3.13g (0.01mol) of 17β-hydroxy-4-androstano[2,3-d]isoxazole into a 100ml three-necked flask, then add 50ml of toluene, stir magnetically, and control the temperature at 0°C. Under temperature, add 45.2mg (0.2mmol) diazepam Schiff base (E)-4--(pyridine-2-amino) benzoic acid, 50mg (0.2mmol) methyl rhenium trioxide and 2.27g (0.02mol) respectively 30%H 2 o 2 Aqueous solution, after stirring for 6 hours, stop the reaction, add a little sodium thiosulfate to remove excess H 2 o 2 , the catalyst was removed by filtration, and the residue was recrystallized with acetone to obtain 2.27g of 17β-hydroxyl-4α,5α-epoxyandrostano[2,3-d]isoxazole after evaporating the solvent, with a yield of 70.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com